2.1. The cuticle
The cuticle is a key contributor to the success of the Insecta. This inert layer provides the strong exoskeleton of body and limbs, the apodemes (internal supports and muscle attachments), and wings, and acts as a barrier between living tissues and the environment. Internally, cuticle lines the tracheal tubes (section 3.5), some gland ducts and the foregut and midgut of the digestive tract. Cuticle may range from rigid and armor-like, as in most adult beetles, to thin and flexible, as in many larvae. Restriction of water loss is a critical function of cuticle vital to the success of insects on land.
The cuticle is thin but its structure is complex and still the subject of some controversy. A single layer of cells, the epidermis, lies beneath and secretes the cuticle, which consists of a thicker procuticle overlaid with thin epicuticle (Fig. 2.1). The epidermis and cuticle together form an integument — the outer covering of the living tissues of an insect.
The epicuticle ranges from 3 µm down to 0.1 µm in thickness, and usually consists of three layers: an inner epicuticle, an outer epicuticle, and a superficial layer. The superficial layer (probably a glycoprotein) in many insects is covered by a lipid or wax layer, sometimes called a free-wax layer, with a variably discrete cement layer external to this. The chemistry of the epicuticle and its outer layers is vital in preventing dehydration, a function derived from water-repelling (hydrophobic) lipids, especially hydrocarbons. These compounds include free and protein-bound lipids, and the outermost waxy coatings give a bloom to the external surface of some insects. Other cuticular patterns, such as light reflectivity, are produced by various kinds of epicuticular surface microsculpturing, such as close-packed, regular or irregular tubercles, ridges, or tiny hairs. Lipid composition can vary and waxiness can increase seasonally or under dry conditions. Besides being water retentive, surface waxes may deter predation, provide patterns for mimicry or camouflage, repel excess rainwater, reflect solar and ultraviolet radiation, or give species-specific olfactory cues.
The epicuticle is inextensible and unsupportive. Instead, support is given by the underlying chitinous cuticle known as procuticle when it is first secreted. This differentiates into a thicker endocuticle covered by a thinner exocuticle, due to sclerotization of the latter. The procuticle is from 10 µm to 0.5 mm thick and consists primarily of chitin complexed with protein. This contrasts with the overlying epicuticle which lacks chitin.
Chitin is found as a supporting element in fungal cell walls and arthropod exoskeletons, and is especially important in insect extracellular structures. It is an unbranched polymer of high molecular weight — an amino-sugar polysaccharide predominantly composed of β-(1-4)-linked units of N-acetyl-d-glucosamine (Fig. 2.2).
Chitin molecules are grouped into bundles and assembled into flexible microfibrils that are embedded in, and intimately linked to, a protein matrix, giving great tensile strength. The commonest arrangement of chitin microfibrils is in a sheet, in which the microfibrils are in parallel. In the exocuticle, each successive sheet lies in the same plane but may be orientated at a slight angle relative to the previous sheet, such that a thickness of many sheets produces a helicoid arrangement, which in sectioned cuticle appears as alternating light and dark bands (lamellae). Thus the parabolic patterns and lamellar arrangement, visible so clearly in sec- tioned cuticle, represent an optical artifact resulting from microfibrillar orientation (Fig. 2.3). In the endocuticle, alternate stacked or helicoid arrangements of microfibrillar sheets may occur, often giving rise to thicker lamellae than in the exocuticle. Different arrangements may be laid down during darkness com- pared with daylight, allowing precise age determination in many adult insects.
Much of the strength of cuticle comes from extensive hydrogen bonding of adjacent chitin chains. Additional stiffening comes from sclerotization, an irreversible process that darkens the exocuticle and results in the proteins becoming water-insoluble. Sclerotization may result from linkages of adjacent protein chains by phenolic bridges (quinone tanning), or from controlled dehydration of the chains, or both. Only exocuticle becomes sclerotized. The deposition of pigment in the cuticle, including deposition of melanin, may be associated with quinones, but is additional to sclerotization and not necessarily associated with it.
In contrast to the solid cuticle typical of sclerites and mouthparts such as mandibles, softer, plastic, highly flexible or truly elastic cuticles occur in insects in varying locations and proportions. Where elastic or spring- like movement occurs, such as in wing ligaments or for the jump of a flea, resilin — a “rubber-like” protein — is present. The coiled polypeptide chains of this protein function as a mechanical spring under tension or compression, or in bending.
In soft-bodied larvae and in the membranes between segments, the cuticle must be tough, but also flexible and capable of extension. This “soft” cuticle, sometimes termed arthrodial membrane, is evident in gravid females, for example in the ovipositing migratory locust, Locusta migratoria (Orthoptera: Acrididae), in which intersegmental membranes may be expanded up to 20-fold for oviposition. Similarly, the gross abdominal dilation of gravid queen bees, termites, and ants is possible through expansion of the unsclerotized cuticle. In these insects, the overlying unstretchable epicuticle expands by unfolding from an originally highly folded state, and some new epicuticle is formed. An extreme example of the distensibility of arthrodial membrane is seen in honeypot ants (Fig. 2.4; see also section 12.2.3). In Rhodnius nymphs (Hemiptera: Reduviidae), changes in molecular structure of the cuticle allow actual stretching of the abdominal membrane to occur in response to intake of a large fluid volume during feeding.
Cuticular structural components, waxes, cements, pheromones (Chapter 4), and defensive and other compounds are products of the epidermis, which is a near- continuous, single-celled layer beneath the cuticle.
Many of these compounds are secreted to the outside of the insect epicuticle. Numerous fine pore canals traverse the procuticle and then branch into numerous finer wax canals (containing wax filaments) within the epicuticle (enlargement in Fig. 2.1); this system transports lipids (waxes) from the epidermis to the epicuticular surface. The wax canals may also have a structural role within the epicuticle. Dermal glands, part of the epidermis, produce cement and/or wax, which is transported via larger ducts to the cuticular surface. Wax-secreting glands are particularly well developed in mealybugs and other scale insects (Fig. 2.5). The epidermis is closely associated with molting — the events and processes leading up to and including ecdysis (eclosion), i.e. the shedding of the old cuticle (section 6.3).
Insects are well endowed with cuticular extensions, varying from fine and hair-like to robust and spine-like. Four basic types of protuberance (Fig. 2.6), all with sclerotized cuticle, can be recognized on morphological, functional, and developmental grounds:
- spines are multicellular with undifferentiated epidermal cells;
- setae, also called hairs, macrotrichia, or trichoid sensilla, are multicellular with specialized cells;
- acanthae are unicellular in origin;
- microtrichia are subcellular, with several to many extensions per cell.
Setae sense much of the insect’s tactile environment. Large setae may be called bristles or chaetae, with the most modified being scales, the flattened setae found on butterflies and moths (Lepidoptera) and sporadically elsewhere. Three separate cells form each seta, one for hair formation (trichogen cell), one for socket formation (tormogen cell), and one sensory cell (Fig. 4.1).
There is no such cellular differentiation in multicellular spines, unicellular acanthae, and subcellular microtrichia. The functions of these types of protuberances are diverse and sometimes debatable, but their sensory function appears limited. The production of pattern, including color, may be significant for some of the microscopic projections. Spines are immovable, but if they are articulated, then they are called spurs. Both spines and spurs may bear unicellular or subcellular processes.
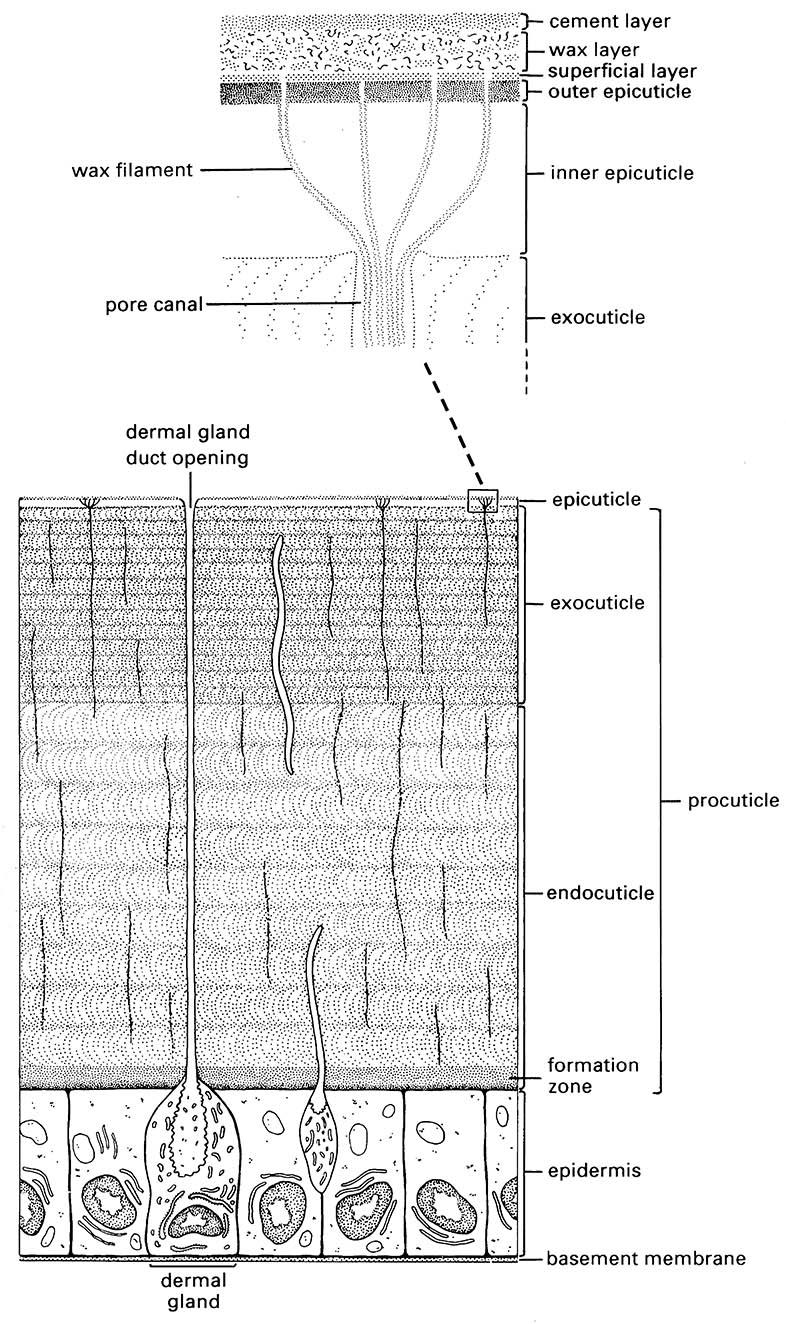
(After Hepburn 1985; Hadley 1986; Binnington 1993)
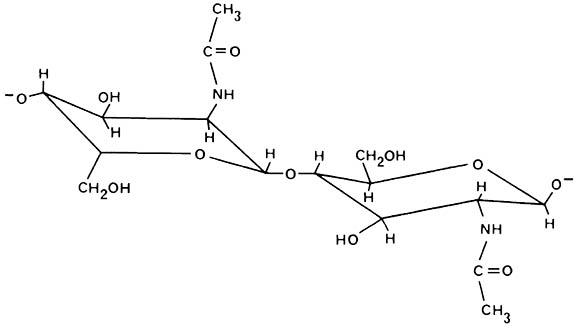
(After Cohen 1991)
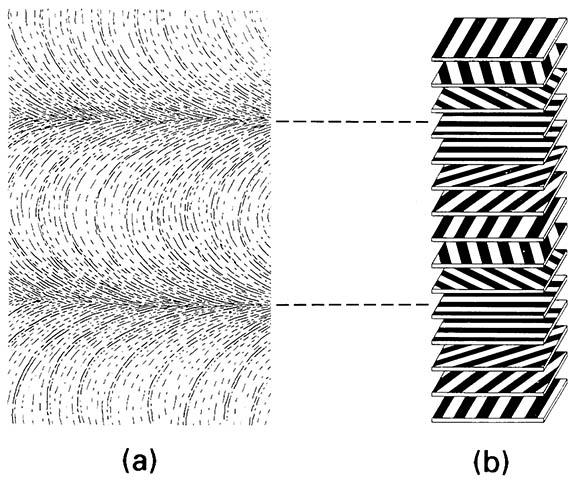
The arrangement of chitin microfibrils in a helicoidal array produces characteristic (though artifactual) parabolic patterns. (b) Diagram of how the rotation of microfibrils produces a lamellar effect owing to microfibrils being either aligned or non-aligned to the plane of sectioning. (After Filshie 1982)
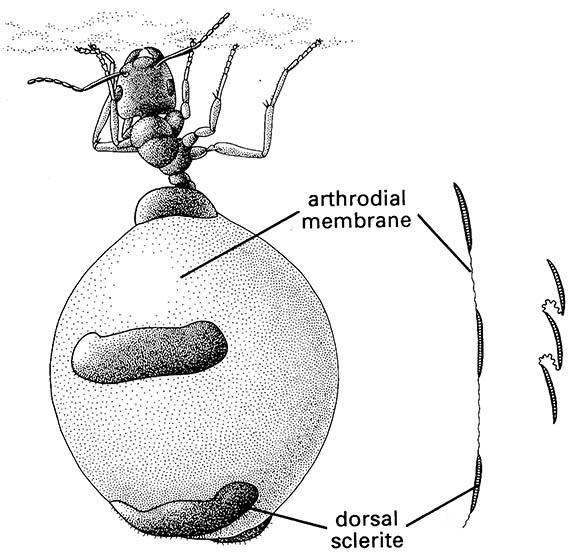
The arthrodial membrane between tergal plates is depicted to the right in its unfolded and folded conditions. (After Hadley 1986; Devitt 1989)
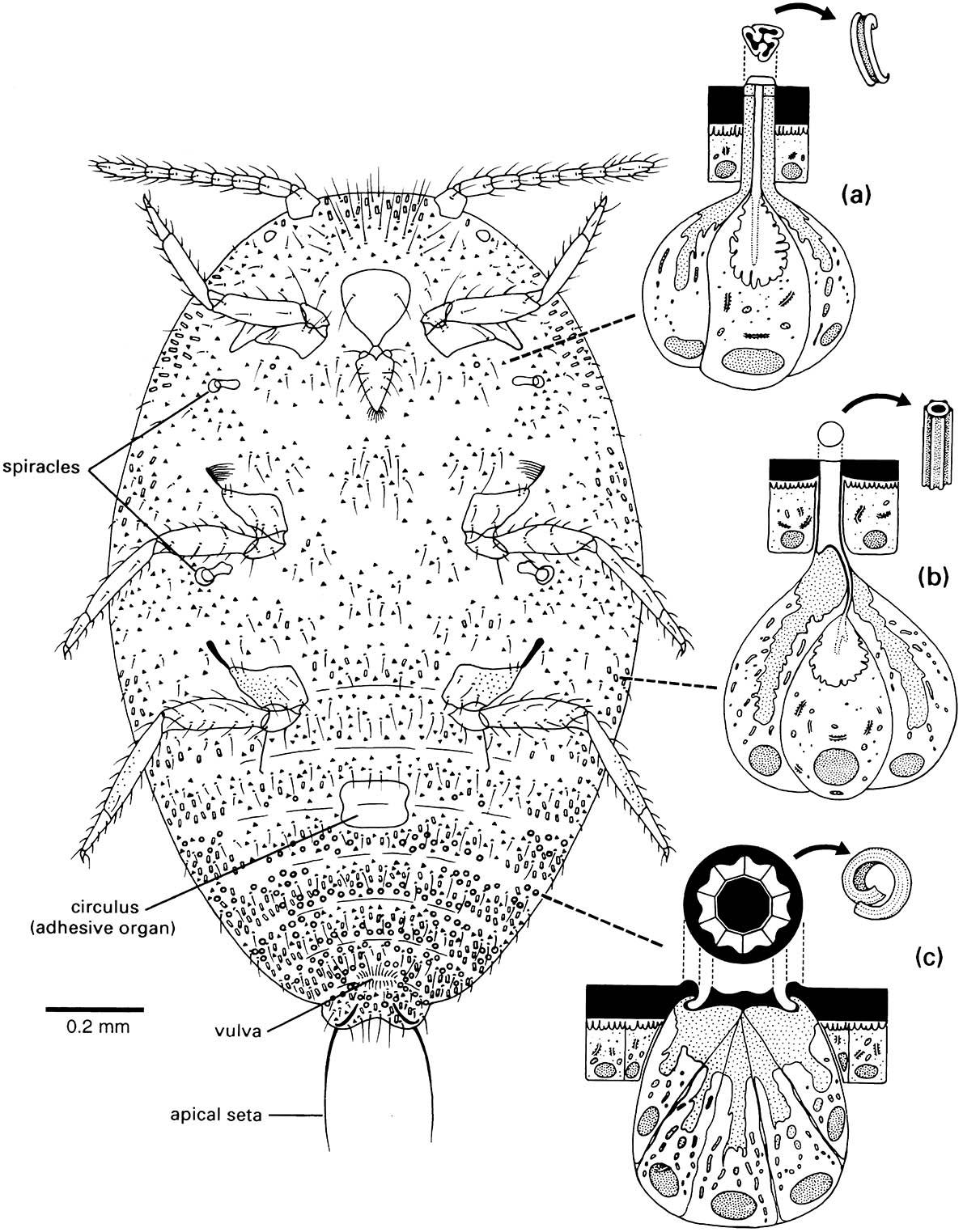
Enlargements depict the ultrastructure of the wax glands and the various wax secretions (arrowed) associated with three types of cuticular structure: (a) a trilocular pore; (b) a tubular duct; and (c) a multilocular pore. Curled filaments of wax from the trilocular pores form a protective body-covering and prevent contamination with their own sugary excreta, or honeydew; long, hollow, and shorter curled filaments from the tubular ducts and multilocular pores, respectively, form the ovisac. (After Foldi 1983; Cox 1987)
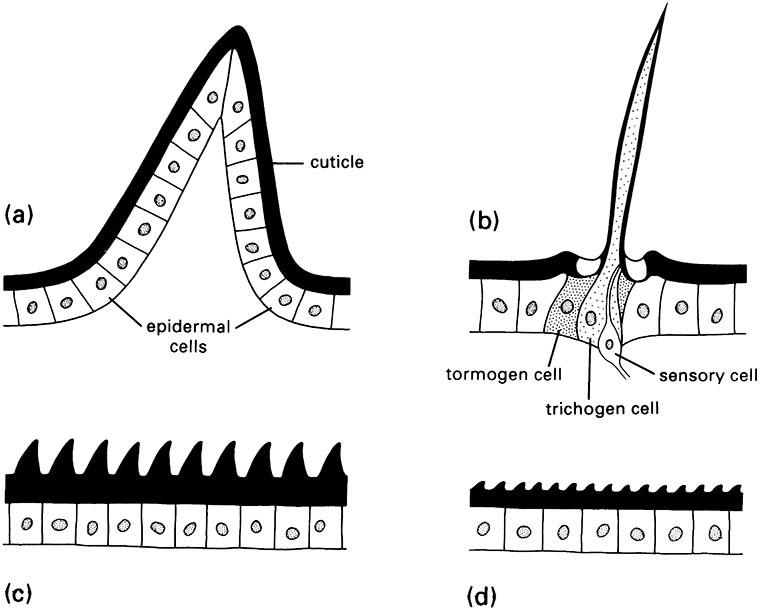
(After Richards & Richards 1979)
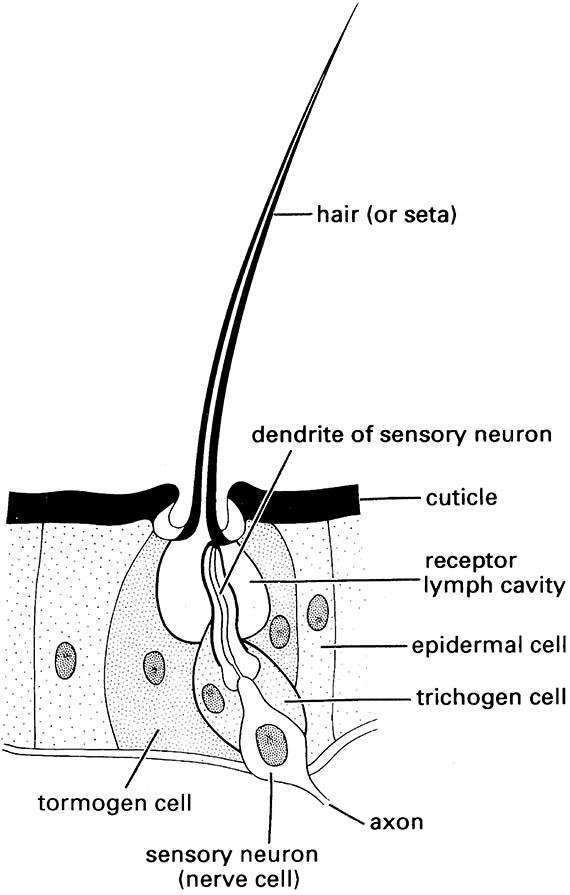
(After Chapman 1991)

