6.3. Process and control of molting
For practical reasons an instar is defined from ecdysis to ecdysis (Fig. 6.1), but morphologically and physiologically a new instar comes into existence at the time of apolysis when the epidermis separates from the cuticle of the previous stage. Apolysis is difficult to detect in most insects but knowledge of its occurrence may be important because many insects spend a substantial period in the pharate state (cloaked within the cuticle of the previous instar) awaiting conditions favorable for emergence as the next stage. Insects often survive adverse conditions as pharate pupae or pharate adults (e.g. some diapausing adult moths) because in this state the double cuticular layer restricts water loss during a developmental period during which metabolism is reduced and requirements for gaseous exchange are minimal.
Molting is a complex process involving hormonal, behavioral, epidermal, and cuticular changes that lead up to the shedding of the old cuticle. The epidermal cells are actively involved in molting — they are responsible for partial breakdown of the old cuticle and formation of the new cuticle. The molt commences with the retraction of the epidermal cells from the inner surface of the old cuticle, usually in an antero-posterior direction. This separation is not total because muscles and sensory nerves retain their connection with the old cuticle. Apolysis is either correlated with or followed by mitotic division of the epidermal cells leading to increases in the volume and surface area of the epidermis. The subcuticular or apolysial space formed after apolysis becomes filled with the secreted but inactive molting fluid. The chitinolytic and proteolytic enzymes of the molting fluid are not activated until the epidermal cells have laid down the protective outer layer of a new cuticle. Then the inner part of the old cuticle (the endocuticle) is lysed and presumably resorbed, while the new pharate cuticle continues to be deposited as an undifferentiated procuticle. Ecdysis commences with the remnants of the old cuticle splitting along the dorsal midline as a result of increase in hemolymph pressure. The cast cuticle consists of the indigestible protein, lipid, and chitin of the old epicuticle and exocuticle. Once free of the constraints of this previous “skin”, the newly ecdysed insect expands the new cuticle by swal-lowing air or water and/or by increasing hemolymph pressure in different body parts to smooth out the wrinkled and folded epicuticle and stretch the procuticle. After cuticular expansion, some or much of the body surface may become sclerotized by the chemical stiffening and darkening of the procuticle to form exocuticle (section 2.1). However, in larval insects most of the body cuticle remains membranous and exocuticle is confined to the head capsule. Following ecdysis, more proteins and chitin are secreted from the epidermal cells thus adding to the inner part of the procuticle, the endocuticle, which may continue to be deposited well into the intermolt period. Sometimes the endocuticle is partially sclerotized during the stadium and frequently the outer surface of the cuticle is covered in wax secretions. Finally, the stadium draws to an end and apolysis is initiated once again.
The above events are effected by hormones acting on the epidermal cells to control the cuticular changes and also on the nervous system to co-ordinate the behaviors associated with ecdysis. Hormonal regulation of molting has been studied most thoroughly at meta- morphosis, when endocrine influences on molting per se are difficult to separate from those involved in the control of morphological change. The classical view of the hormonal regulation of molting and metamorpho- sis is presented schematically in Fig. 6.9; the endocrine centers and their hormones are described in more detail in Chapter 3. Three major types of hormones control molting and metamorphosis:
- neuropeptides, including prothoracicotropic hor- mone (PTTH), ETH, and EH;
- ecdysteroids;
- juvenile hormone (JH), which may occur in several different forms even in the same insect.
Neurosecretory cells in the brain secrete PTTH, which passes down nerve axons to the corpora allata, a pair of neuroglandular bodies that store and later release PTTH into the hemolymph. The PTTH stimulates ecdysteroid synthesis and secretion by the prothoracic or molting glands. Ecdysteroid release then initiates the changes in the epidermal cells that lead to the production of new cuticle. The characteristics of the molt are regulated by JH from the corpora allata; JH inhibits the expression of adult features so that a high hemolymph level (titer) of JH is associated with a larval—larval molt, and a lower titer with a larval—pupal molt; JH is absent at the pupal—adult molt.
Ecdysis is mediated by ETH and EH, and EH at least appears to be important at every molt in the life history of perhaps all insects. This neuropeptide acts on a steroid-primed central nervous system to evoke the co-ordinated motor activities associated with escape from the old cuticle. Eclosion hormone derives its name from the pupal—adult ecdysis, or eclosion, for which its importance was first discovered and before its wider role was realized. Indeed, the association of EH with molting appears to be ancient, as other arthropods (e.g. crustaceans) have EH homologues. In the well-studied tobacco hornworm (section 6.2.4), the more recently discovered ETH is as important to ecdysis as EH, with ETH and EH stimulating each other’s release, but the taxonomic distribution of ETH is not yet known. In many insects, another neuropeptide, bursicon, controls sclerotization of the exocuticle and postmolt deposition of endocuticle.
The relationship between the hormonal environment and the epidermal activities that control molting and cuticular deposition in a lepidopteran, the tobacco hornworm Manduca sexta, are presented in Fig. 6.10.
Only now are we beginning to understand how hormones regulate molting and metamorphosis at the cellular and molecular levels. However, detailed studies on the tobacco hornworm clearly show the correlation between the ecdysteroid and JH titers and the cuticular changes that occur in the last two larval instars and in prepupal development. During the molt at the end of the fourth larval instar, the epidermis responds to the surge of ecdysteroid by halting synthesis of endocuticle and the blue pigment insecticyanin. A new epicuticle is synthesized, much of the old cuticle is digested, and resumption of endocuticle and insecticyanin production occurs by the time of ecdysis. In the final larval instar the JH declines to undetectable levels, allowing small rises in ecdysteroid that first stimulate the epidermis to produce a stiffer cuticle with thinner lamellae and then elicit wandering in the larva. When ecdysteroid initiates the next molt, the epidermal cells produce pupal cuticle as a result of the activation of many new genes. The decline in ecdysteroid level towards the end of each molt seems to be essential for, and may be the physiological trigger causing, ecdysis to occur. It renders the tissues sensitive to EH and permits the release of EH into the hemolymph (see section 6.2.4 for further discussion of the actions of eclosion hormone). Apolysis at the end of the fifth larval instar marks the beginning of a prepupal period when the developing pupa is pharate within the larval cuticle. Differentiated exocuticle and endocuticle appear at this larval—pupal molt. During larval life, the epidermal cells covering most of the body do not produce exocuticle, so the caterpillar’s cuticle is soft and flexible allowing considerable growth within an instar as a result of feeding.
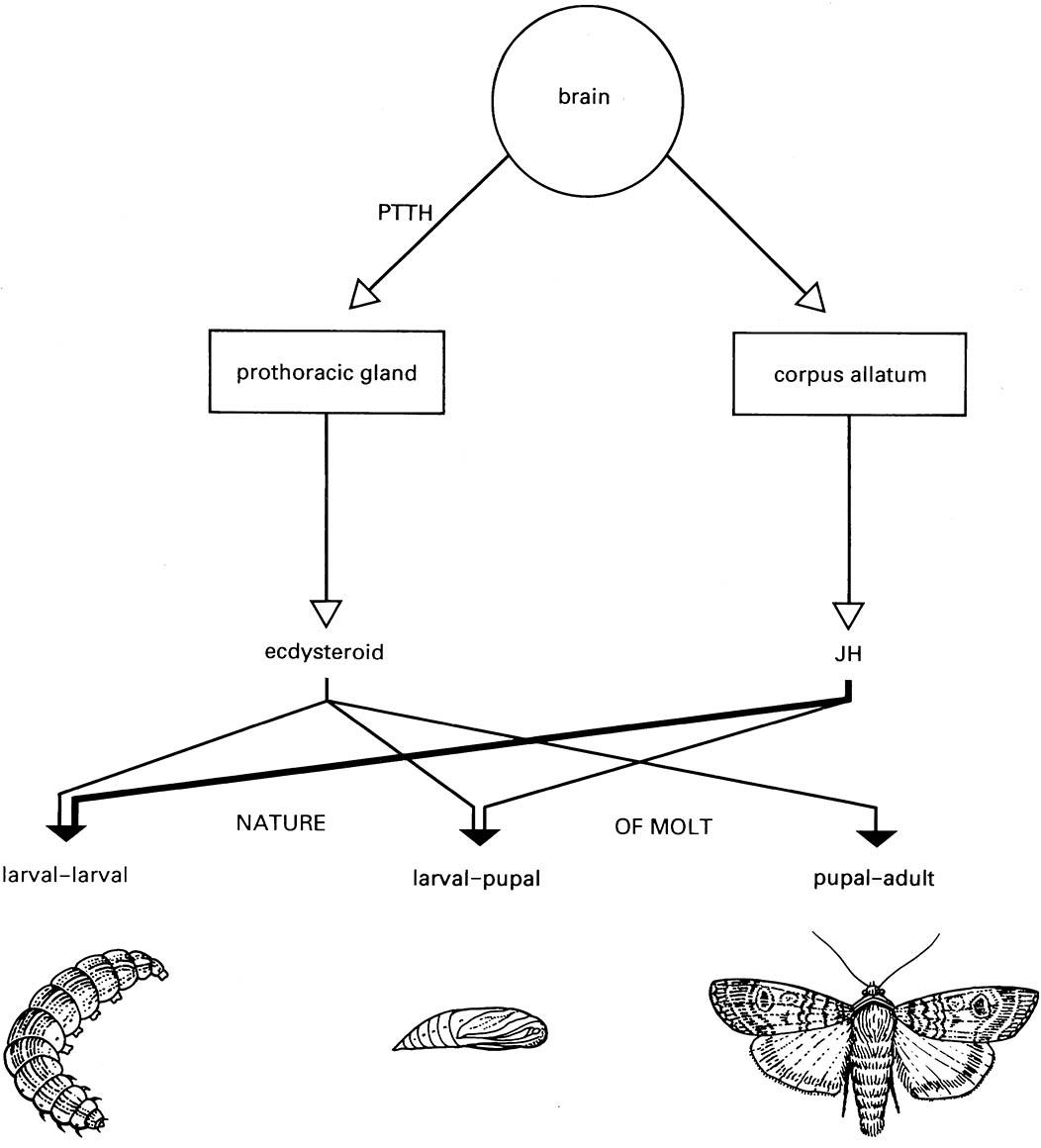
This scheme simplifies the complexity of ecdysteroid and JH secretion and does not indicate the influence of neuropeptides such as eclosion hormone. JH, juvenile hormone; PTTH, prothoracicotropic hormone. (After Richards 1981)

The dots in the epidermal cells represent granules of the blue pigment insecticyanin. ETH, ecdysis triggering hormone; EH, eclosion hormone; JH, juvenile hormone; EPI, EXO, ENDO, deposition of pupal epicuticle, exocuticle, and endocuticle, respectively. The numbers on the x-axis represent days. (After Riddiford 1991)

