2.3.1. Mouthparts
The mouthparts are formed from appendages of all head segments except the second. In omnivorous insects, such as cockroaches, crickets, and earwigs, the mouthparts are of a biting and chewing type (mandibulate) and resemble the probable basic design of ancestral pterygote insects more closely than the mouthparts of the majority of modern insects. Extreme modifications of basic mouthpart structure, correlated with feeding specializations, occur in most Lepidoptera, Diptera, Hymenoptera, Hemiptera, and a number of the smaller orders. Here we first discuss basic mandibulate mouthparts, as exemplified by the European earwig, Forficula auricularia (Dermaptera: Forficulidae) (Fig. 2.10), and then describe some of the more common modifications associated with more specialized diets.
There are five basic components of the mouthparts:
- labrum, or “upper lip”, with a ventral surface called the epipharynx;
- hypopharynx, a tongue-like structure;
- mandibles, or jaws;
- maxillae (singular: maxilla);
- labium, or “lower lip” (Fig. 2.10).
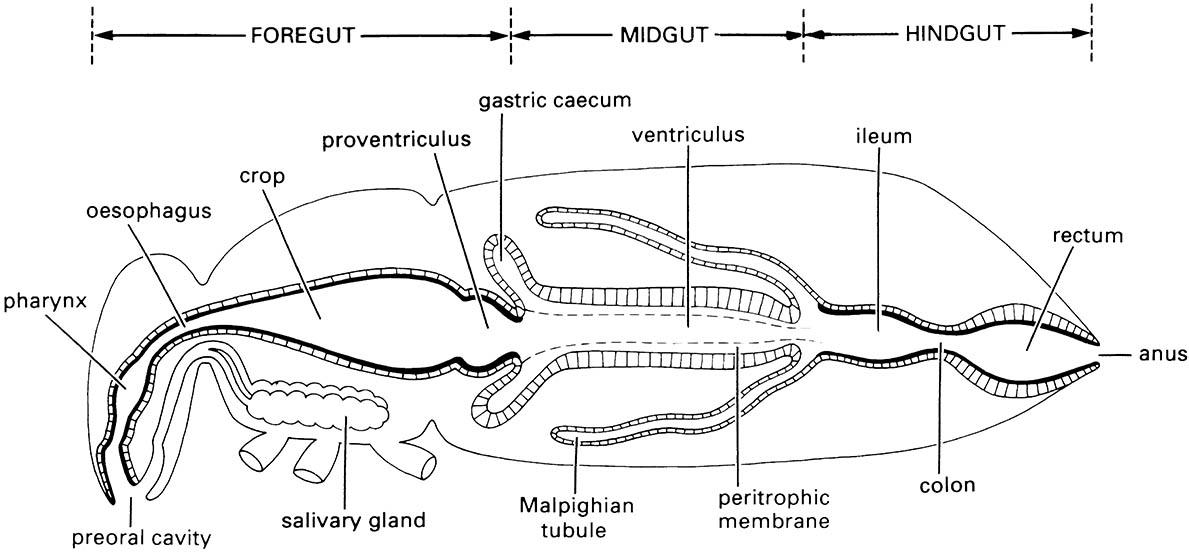
The labrum forms the roof of the preoral cavity and mouth (Fig. 3.14) and covers the base of the mandibles; it may be formed from fusion of parts of a pair of ancestral appendages. Projecting forwards from the back of the preoral cavity is the hypopharynx, a lobe of probable composite origin; in apterygotes, earwigs, and nymphal mayflies the hypopharynx bears a pair of lateral lobes, the superlinguae (singular: superlingua) (Fig. 2.10). It divides the cavity into a dorsal food pouch, or cibarium, and a ventral salivarium into which the salivary duct opens (Fig. 3.14). The mandibles, maxillae, and labium are the paired appendages of segments 4–6 and are highly variable in structure among insect orders; their serial homology with walking legs is more apparent than for the labrum and hypopharynx.
The mandibles cut and crush food and may be used for defense; generally they have an apical cutting edge and the more basal molar area grinds the food. They can be extremely hard (approximately 3 on Moh’s scale of mineral hardness, or an indentation hardness of about 30 kg mm-2) and thus many termites and beetles have no physical difficulty in boring through foils made from such common metals as copper, lead, tin, and zinc. Behind the mandibles lie the maxillae, each consisting of a basal part composed of the proximal cardo and the more distal stipes and, attached to the stipes, two lobes — the mesal lacinia and the lateral galea — and a lateral, segmented maxillary palp, or palpus (plural: palps or palpi). Functionally, the maxillae assist the mandibles in processing food; the pointed and sclerotized lacinae hold and macerate the food, whereas the galeae and palps bear sensory setae (mechanoreceptors) and chemoreceptors which sample items before ingestion. The appendages of the sixth segment of the head are fused with the sternum to form the labium, which is believed to be homologous to the second maxillae of Crustacea. In prognathous insects, such as the earwig, the labium attaches to the ventral surface of the head via a ventromedial sclerotized plate called the gula (Fig. 2.10). There are two main parts to the labium: the proximal postmentum, closely connected to the posteroventral surface of the head and sometimes subdivided into a submentum and mentum; and the free distal prementum, typically bearing a pair of labial palps lateral to two pairs of lobes, the mesal glossae (singular: glossa) and the more lateral paraglossae (singular: paraglossa). The glossae and paraglossae, including sometimes the distal part of the prementum to which they attach, are known collectively as the ligula; the lobes may be variously fused or reduced as in Forficula (Fig. 2.10), in which the glossae are absent. The prementum with its lobes forms the floor of the preoral cavity (functionally a “lower lip”), whereas the labial palps have a sensory function, similar to that of the maxillary palps.
During insect evolution, an array of different mouthpart types have been derived from the basic design described above. Often feeding structures are characteristic of all members of a genus, family, or order of insects, so that knowledge of mouthparts is useful for both taxonomic classification and identification, and for ecological generalization (see section 10.6). Mouthpart structure is categorized generally according to feeding method, but mandibles and other components may function in defensive combat or even male—male sexual contests, as in the enlarged mandibles on certain male beetles (Lucanidae). Insect mouthparts have diversified in different orders, with feeding methods that include lapping, suctorial feeding, biting, or piercing combined with sucking, and filter feeding, in addition to the basic chewing mode.
The mouthparts of bees are of a chewing and lapping type. Lapping is a mode of feeding in which liquid or semi-liquid food adhering to a protrusible organ, or “tongue”, is transferred from substrate to mouth. In the honey bee, Apis mellifera (Hymenoptera: Apidae), the elongate and fused labial glossae form a hairy tongue, which is surrounded by the maxillary galeae and the labial palps to form a tubular proboscis containing a food canal (Fig. 2.11). In feeding, the tongue is dipped into the nectar or honey, which adheres to the hairs, and then is retracted so that adhering liquid is carried into the space between the galeae and labial palps. This back-and-forth glossal movement occurs repeatedly. Movement of liquid to the mouth apparently results from the action of the cibarial pump, facilitated by each retraction of the tongue pushing liquid up the food canal. The maxillary laciniae and palps are rudimentary and the paraglossae embrace the base of the tongue, directing saliva from the dorsal salivary orifice around into a ventral channel from whence it is transported to the flabellum, a small lobe at the glossal tip; saliva may dissolve solid or semi-solid sugar. The sclerotized, spoon-shaped mandibles lie at the base of the proboscis and have a variety of functions, including the manipulation of wax and plant resins for nest construction, the feeding of larvae and the queen, grooming, fighting, and the removal of nest debris including dead bees.
Most adult Lepidoptera and some adult flies obtain their food solely by sucking up liquids using suctorial (haustellate) mouthparts that form a proboscis or rostrum (Box 15.5). Pumping of the liquid food is achieved by muscles of the cibarium and/or pharynx. The proboscis of moths and butterflies, formed from the greatly elongated maxillary galeae, is extended (Fig. 2.12a) by increases in hemolymph (“blood”) pressure. It is loosely coiled by the inherent elasticity of the cuticle, but tight coiling requires contraction of intrinsic muscles (Fig. 2.12b). A cross-section of the proboscis (Fig. 2.12c) shows how the food canal, which opens basally into the cibarial pump, is formed by apposition and interlocking of the two galeae. The proboscis of some male hawk- moths (Sphingidae), such as that of Xanthopan morgani, can attain great length (Fig. 11.8).
A few moths and many flies combine sucking with piercing or biting. For example, moths that pierce fruit and exceptionally suck blood (species of Noctuidae) have spines and hooks at the tip of their proboscis which are rasped against the skins of either ungulate mammals or fruit. For at least some moths, penetration is effected by the alternate protraction and retraction of the two galeae that slide along each other. Bloodfeeding flies have a variety of skin-penetration and feeding mechanisms. In the “lower” flies such as mosquitoes and black flies, and the Tabanidae (horse flies, Brachycera), the labium of the adult fly forms a non-piercing sheath for the other mouthparts, which together contribute to the piercing structure. In contrast, the biting calyptrate dipterans (Brachycera: Calyptratae, e.g. stable flies and tsetse flies) lack mandibles and maxillae and the principal piercing organ is the highly modified labium. Mouthparts of adult Diptera are described in Box 15.5.
Other mouthpart modifications for piercing and sucking are seen in the true bugs (Hemiptera), thrips (Thysanoptera), fleas (Siphonaptera), and sucking lice (Phthiraptera: Anoplura). In each order different mouthpart components form needle-like stylets capable of piercing the plant or animal tissues upon which the insect feeds. Bugs have extremely long, thin paired mandibular and maxillary stylets, which fit together to form a flexible stylet-bundle containing a food canal and a salivary canal (Box 11.8). Thrips have three stylets — paired maxillary stylets (laciniae) plus the left mandibular one (Fig. 2.13). Sucking lice have three stylets — the hypopharyngeal (dorsal), the salivary (median), and the labial (ventral) — lying in a ventral sac of the head and opening at a small eversible proboscis armed with internal teeth that grip the host during blood-feeding (Fig. 2.14). Fleas possess a single stylet derived from the epipharynx, and the laciniae of the maxillae form two long cutting blades that are ensheathed by the labial palps (Fig. 2.15). The Hemiptera and the Thysanoptera are sister groups and belong to the same assemblage as the Phthiraptera (Fig. 7.2), but the lice at least had a psocopteroid-like ancestor, presumably with mouthparts of a more generalized, mandibulate type. The Siphonaptera are distant relatives of the other three taxa; thus similarities in mouthpart structure among these orders result largely from parallel or, in the case of fleas, convergent evolution.
Slightly different piercing mouthparts are found in antlions and the predatory larvae of other lacewings (Neuroptera). The stylet-like mandible and maxilla on each side of the head fit together to form a sucking tube (Fig. 13.2c), and in some families (Chrysopidae, Myrmeleontidae, and Osmylidae) there is also a narrow poison channel. Generally, labial palps are present, maxillary palps are absent, and the labrum is reduced. Prey is seized by the pointed mandibles and maxillae, which are inserted into the victim; its body contents are digested extra-orally and sucked up by pumping of the cibarium.
A unique modification of the labium for prey capture occurs in nymphal damselflies and dragonflies (Odonata These predators catch other aquatic organisms by extending their folded labium (or “mask”) rapidly and seizing mobile prey using prehensile apical hooks on modified labial palps (Fig. 13.4). The labium is hinged between the prementum and postmentum and, when folded, covers most of the underside of the head. Labial extension involves the sudden release of energy, produced by increases in blood pressure brought about by the contraction of thoracic and abdominal muscles, and stored elastically in a cuticular click mechanism at the prementum—postmentum joint. As the click mechanism is disengaged, the elevated hydraulic pressure shoots the labium rapidly forwards. Labial retraction then brings the captured prey to the other mouthparts for maceration.
Filter feeding in aquatic insects has been studied best in larval mosquitoes (Diptera: Culicidae), black flies (Diptera: Simuliidae), and net-spinning caddisflies (Trichoptera: many Hydropsychoidea and Philopotamoidea), which obtain their food by filtering particles (including bacteria, microscopic algae, and detritus) from the water in which they live. The mouthparts of the dipteran larvae have an array of setal “brushes” and/or “fans”, which generate feeding currents or trap particulate matter and then move it to the mouth. In contrast, the caddisflies spin silk nets that filter particulate matter from flowing water and then use their mouthpart brushes to remove particles from the nets. Thus insect mouthparts are modified for filter feeding chiefly by the elaboration of setae. In mosquito larvae the lateral palatal brushes on the labrum generate the feeding currents (Fig. 2.16); they beat actively, causing particle-rich surface water to flow towards the mouth- parts, where setae on the mandibles and maxillae help to move particles into the pharynx, where food boluses form at intervals.
In some adult insects, such as mayflies (Ephemeroptera), some Diptera (warble flies), a few moths (Lepidoptera), and male scale insects (Hemiptera: Coccoidea), mouthparts are greatly reduced and non- functional. Atrophied mouthparts correlate with short adult lifespan.
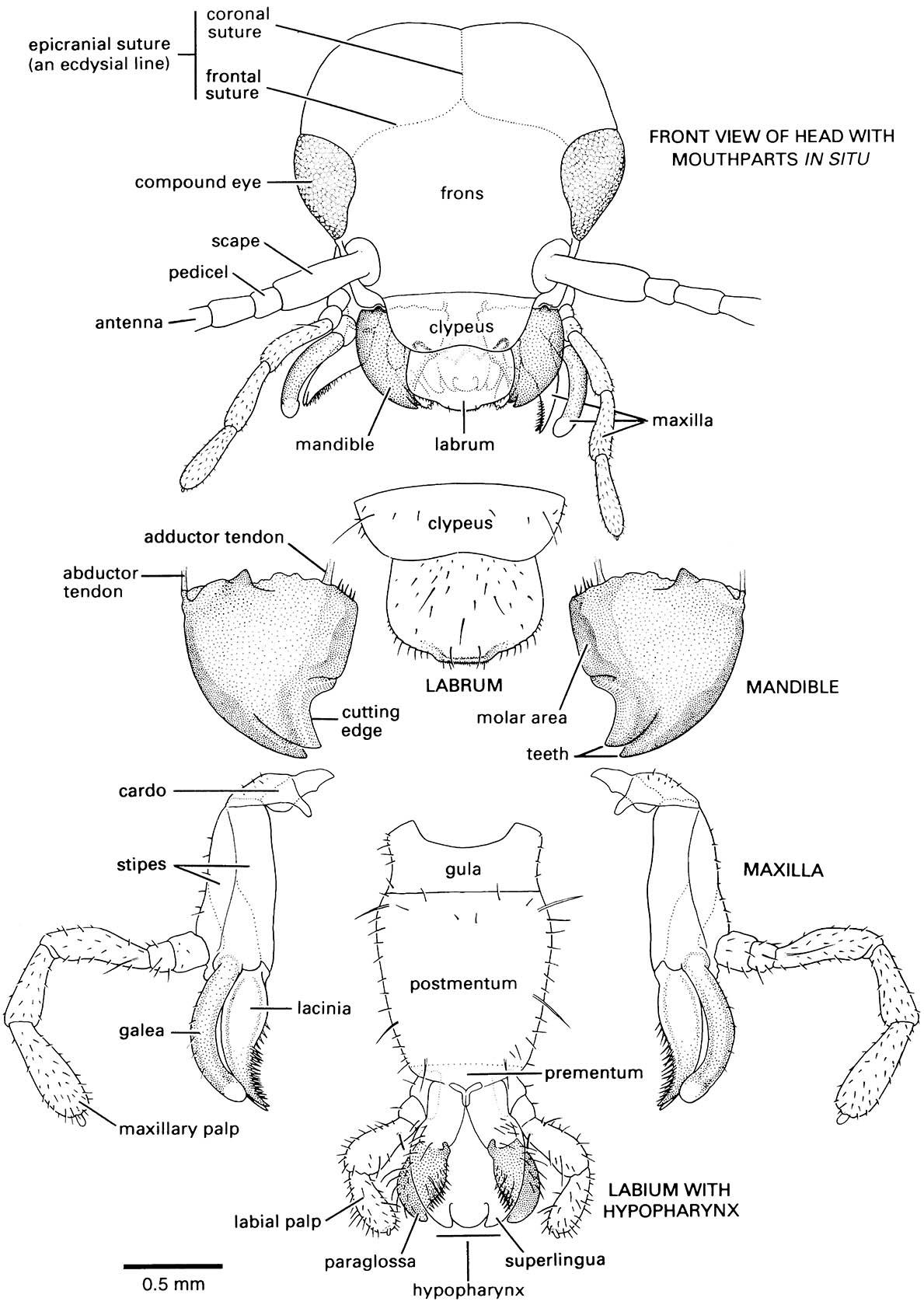
Note that the head is prognathous and thus a gular plate, or gula, occurs in the ventral neck region.
Musculature of the mouthparts and the (a) pharyngeal or (b) cibarial pump are indicated but not fully labeled. Contraction of the respective dilator muscles causes dilation of the pharynx or cibarium and fluid is drawn into the pump chamber. Relaxation of these muscles results in elastic return of the pharynx or cibarial walls and expels food upwards into the oesophagus. (After Snodgrass 1935)

(Inset after Wigglesworth 1964)
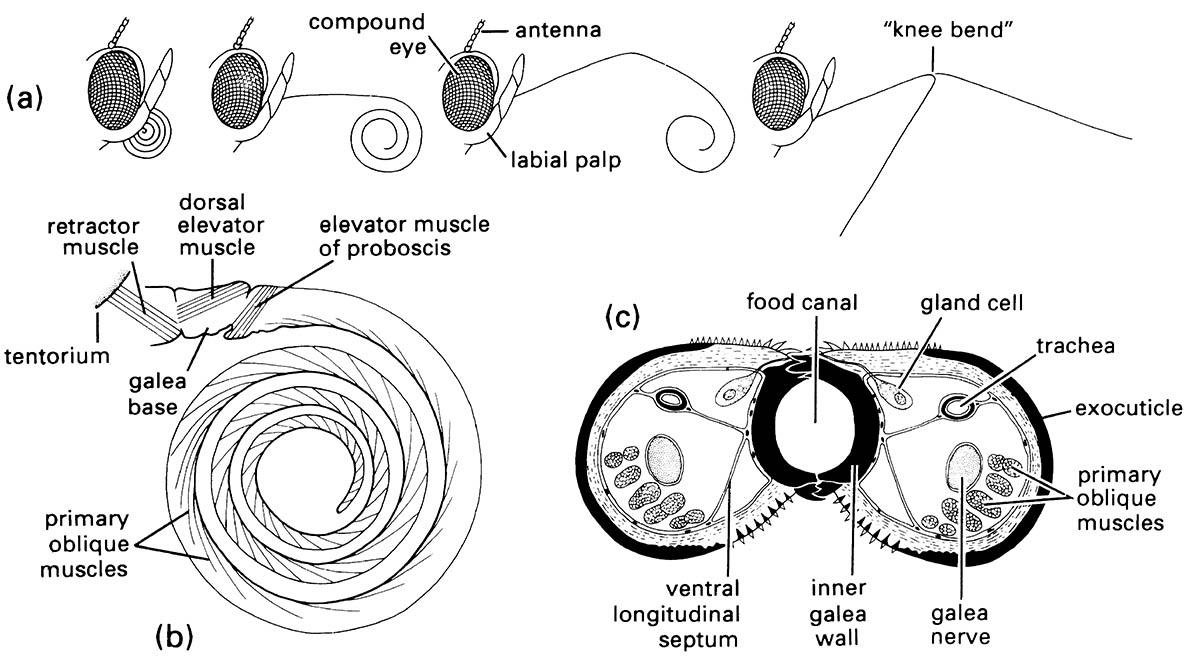
(a) Positions of the proboscis showing, from left to right, at rest, with proximal region uncoiling, with distal region uncoiling, and fully extended with tip in two of many possible different positions due to flexing at «knee bend». (b) Lateral view of proboscis musculature. (c) Transverse section of the proboscis in the proximal region. (After Eastham & Eassa 1955)
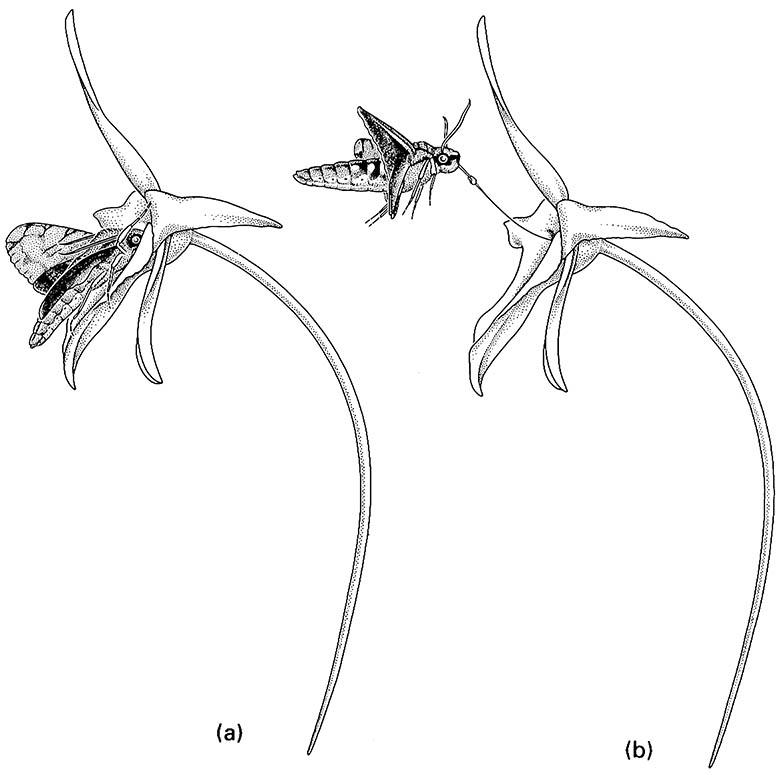
(a) full insertion of the moth’s proboscis; (b) upward flight during withdrawal of the proboscis with the orchid pollinium attached. (After Wasserthal 1997)
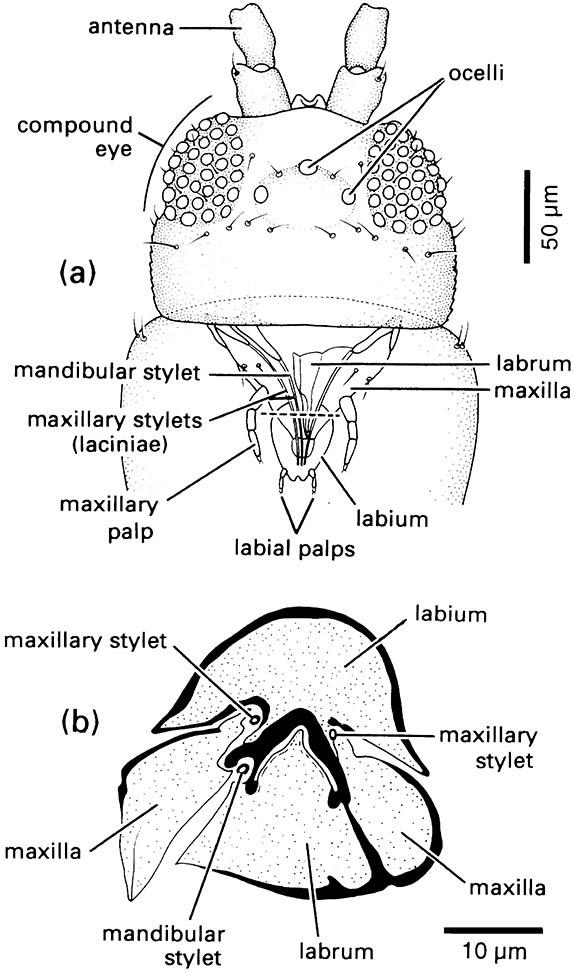
(a) Dorsal view of head showing mouthparts through prothorax. (b) Transverse section through proboscis. The plane of the transverse section is indicated by the dashed line in (a). (After Matsuda 1965; CSIRO 1970)
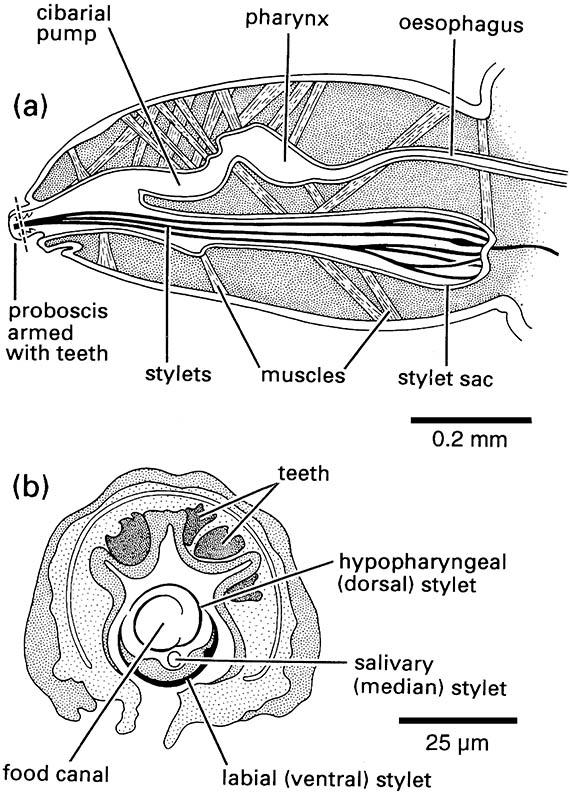
(a) Longitudinal section of head (nervous system omitted). (b) Transverse section through eversible proboscis. The plane of the transverse section is indicated by the dashed line in (a). (After Snodgrass 1935)
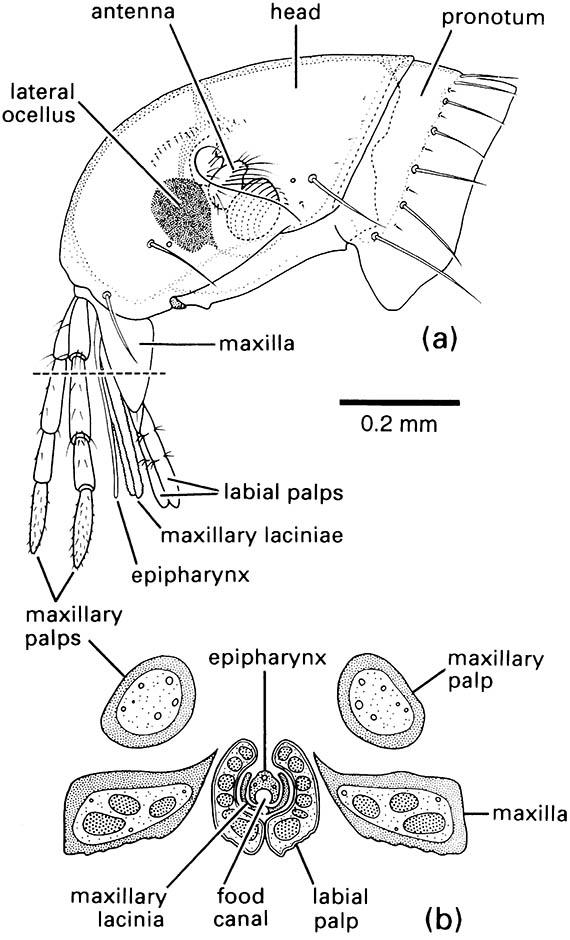
(a) lateral view of head; (b) transverse section through mouthparts. The plane of the transverse section is indicated by the dashed line in (a). (After Snodgrass 1946; Herms & James 1961)
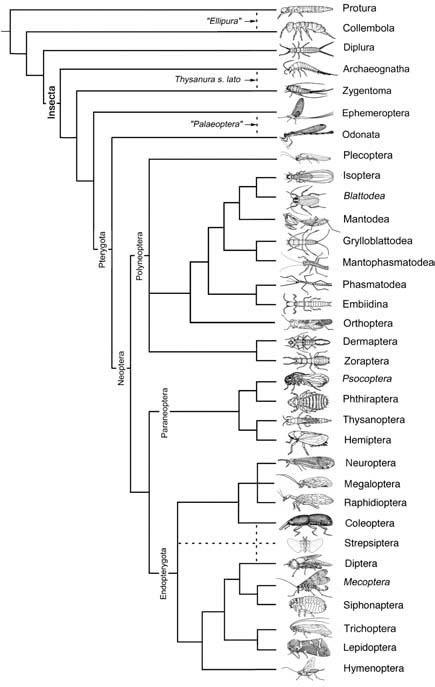
Broken lines indicate uncertain relationships. Thysanura sensu lato refers to Thysanura in the broad sense. (Data from several sources)
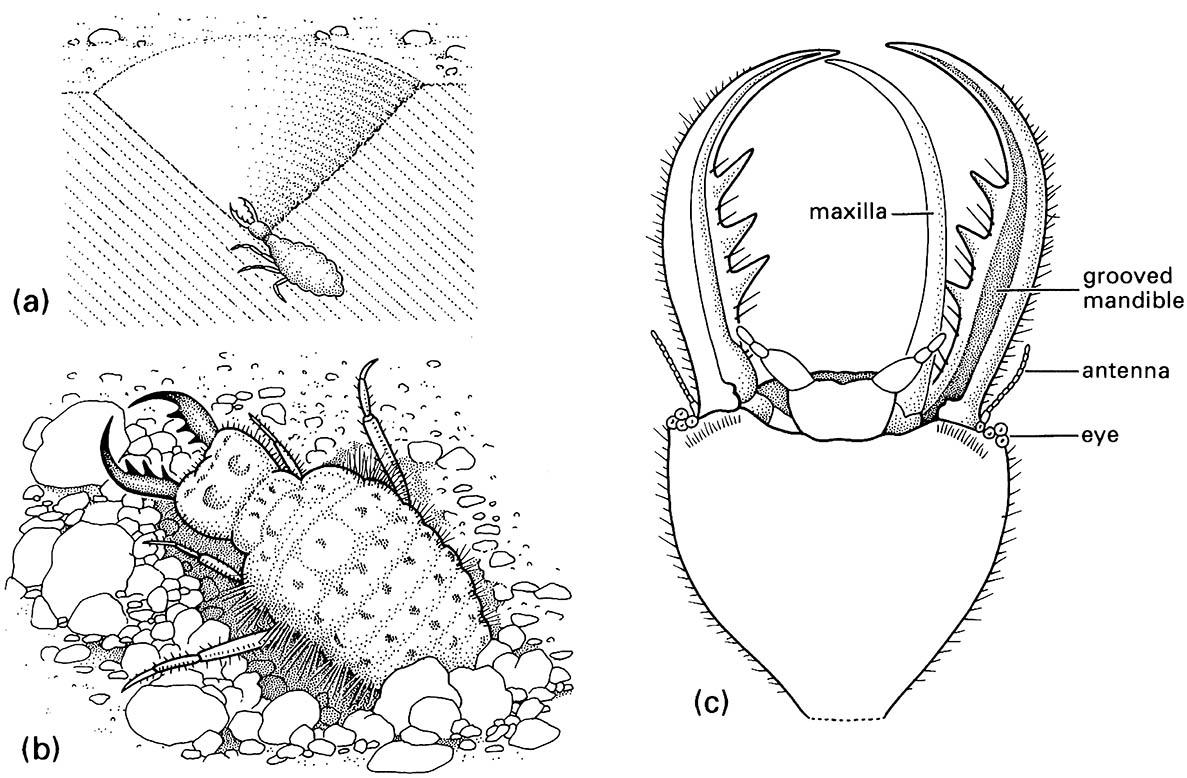
(a) larva in its pit in sand; (b) detail of dorsum of larva; (c) detail of ventral view of larval head showing how the maxilla fits against the grooved mandible to form a sucking tube. (After Wigglesworth 1964)
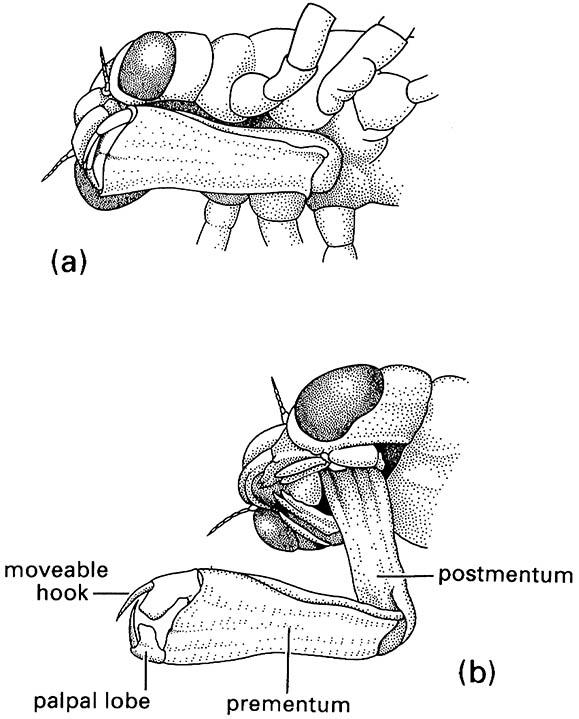
(a) in folded position, and (b) extended during prey capture with opposing hooks of the palpal lobes forming claw-like pincers. (After Wigglesworth 1964)
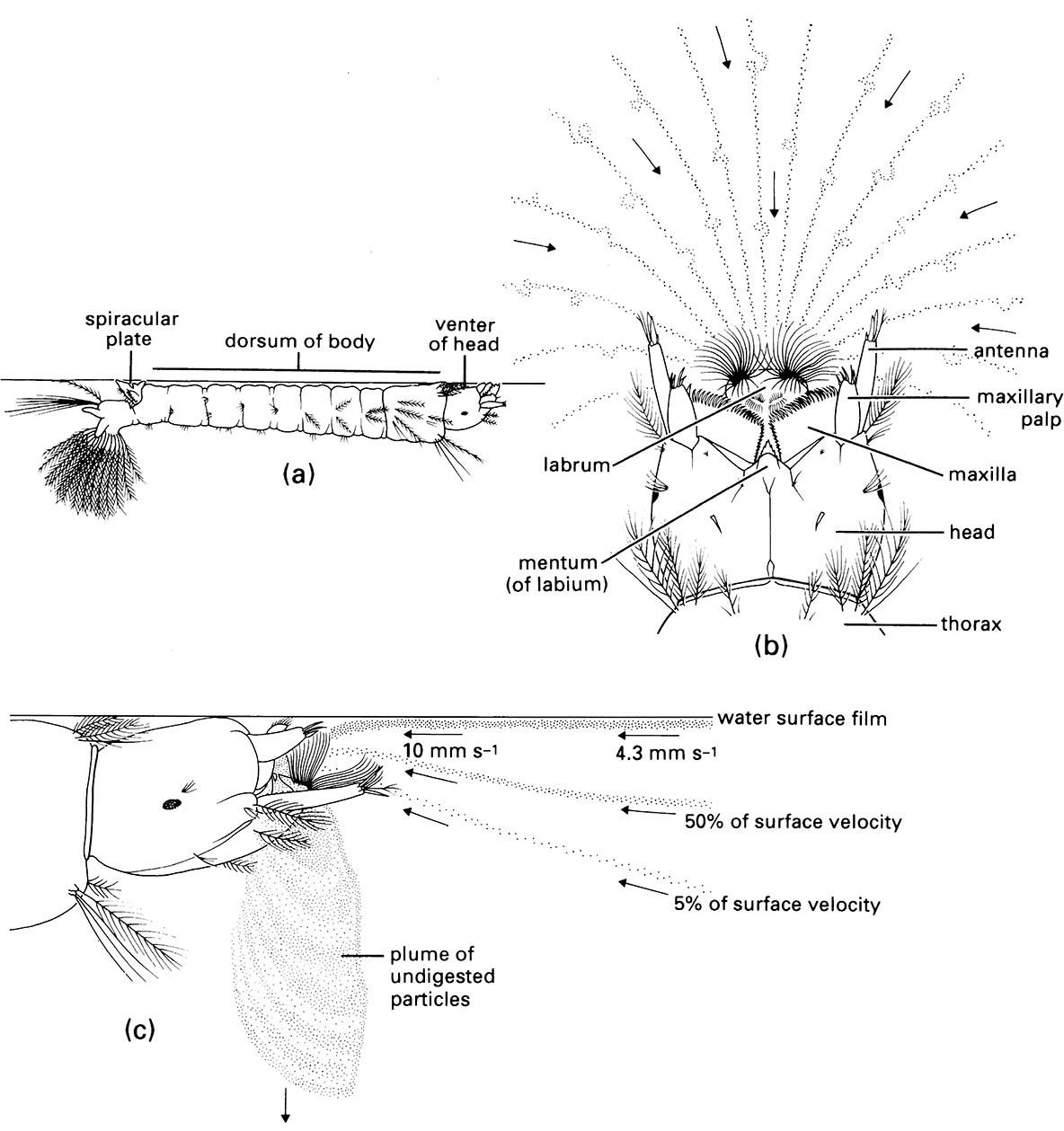
(a) The larva floating just below the water surface, with head rotated through 180° relative to its body (which is dorsum-up so that the spiracular plate near the abdominal apex is in direct contact with the air). (b) Viewed from above showing the venter of the he ad and the feeding current generated by setal brushes on the labrum (direction of water movement and paths taken by surface particles are indicated by arrows and dotted lines, respectively). (c) Lateral view showing the particle-rich water being drawn into the preoral cavity between the mandibles and maxillae and its downward expulsion as the outward current. ((b, c) After Merritt et al. 1992)

