6.10.5. Biotic effects
In most insect orders, adult size has a strong genetic component and growth is strongly determinate. In many Lepidoptera, for example, final adult size is relatively constant within a species; reduction in food quality or availability delays caterpillar growth rather than causing reduced final adult size, although there are exceptions. In contrast, in flies that have limited or ephemeral larval resources, such as a dung pat or temporary pool, cessation of larval growth would result in death as the habitat shrinks. Thus larval crowding and/or limitation of food supply tend to shorten development time and reduce final adult size. In some mosquitoes and midges, success in short-lived pool habitats is attained by a small proportion of the larval population developing with extreme rapidity relative to their slower siblings. In pedogenetic gall midges (section 5.10.1), crowding with reduced food supply terminates larva-only reproductive cycles and induces the production of adults, allowing dispersal to more favorable habitats.
Food quality appears important in all these cases, but there may be related effects, for example as a result of crowding. Clearly, it can be difficult to segregate out food effects from other potentially limiting factors. In the California red scale, Aonidiella aurantii (Hemiptera: Diaspididae), development and reproduction on orange trees is fastest on fruit, intermediate on twigs, and slow- est on leaves. Although these differences may reflect differing nutritional status, a microclimatic explanation cannot be excluded, as fruit may retain heat longer than the relatively smaller-volumed stems and leaves, and such slight temperature differences might affect the development of the insects.
The effects of crowding on development are well understood in some insects, as in locusts in which two extreme phases, termed solitary and gregarious (Fig. 6.13), differ in morphometrics, color, and behavior. At low densities locusts develop into the solitary phase, with a characteristic uniform-colored “hopper” (nymph), and large-sized adult with large hind femora. As densities increase, induced in nature by high survivorship of eggs and young nymphs under favor- able climatic conditions, graded changes occur and a darker-striped nymph develops to a smaller locust with shorter hind femora. The most conspicuous difference is behavioral, with more solitary individuals shunning each other’s company but making concerted nocturnal migratory movements that result eventually in aggregations in one or a few places of gregarious individuals, which tend to form enormous and mobile swarms. The behavioral shift is induced by crowding, as can be shown by splitting a single locust egg pod into two: rearing the offspring at low densities induces solitary locusts, whereas their siblings reared under crowded conditions develop into gregarious locusts. The response to high population density results from the integration of several cues, including the sight, touch, and some- times the odor (pheromone) of conspecifics, which lead to endocrine and neuroendocrine (ecdysteroid) changes associated with developmental transformation.
Under certain circumstances biotic effects can override growth factors. Across much of the eastern USA, 13- and 17-year periodic cicadas (Magicicada spp.) emerge highly synchronously. At any given time, nymphal cicadas are of various sizes and in different instars according to the nutrition they have obtained from feeding on the phloem from roots of a variety of trees. Whatever their growth condition, after the elapse of 13 or 17 years since the previous emergence and egg-laying, the final molt of all nymphs prepares them for synchronous emergence as adults. In a very clever experiment host plants were induced to flush twice in one year, inducing adult cicada emergence one year early compared to controls on the roots of single- flushing trees. This implies that synchronized timing for cicadas depends on an ability to “count off” annual events — the predictable flush of sap with the passing of each spring once a year (except when experimenters manipulate it!).
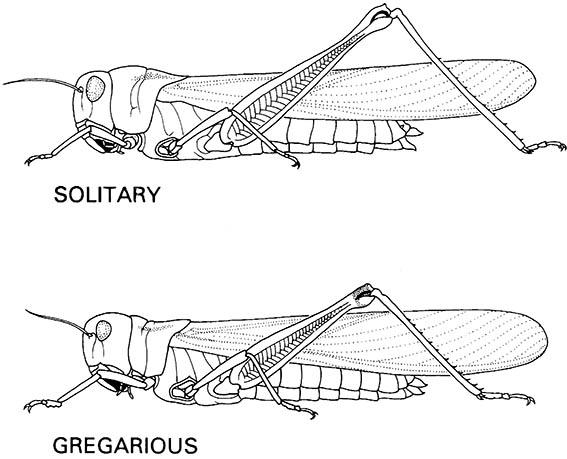
The solitaria adults have a pronounced pronotal crest and the femora are larger relative to the body and wing than in the gregaria adults. Intermediate morphologies occur in the transiens (transient stage) during the transformation from solitaria to gregaria or the reverse.

