4.3.1. Chemoreception
The chemical senses may be divided into taste, for detection of aqueous chemicals, and smell, for air- borne ones — but the distinction is relative. Alternative terms are contact (taste, gustatory) and distant (smell, olfactory) chemoreception. For aquatic insects, all chemicals sensed are in aqueous solution, and strictly all chemoreception should be termed “taste”. However, if an aquatic insect has a chemoreceptor that is structurally and functionally equivalent to one in a terrestrial insect that is olfactory, then the aquatic insect is said to “smell” the chemical.
Chemosensors trap chemical molecules, which are transferred to a site for recognition, where they specifically depolarize a membrane and stimulate a nerve impulse. Effective trapping involves localization of the chemoreceptors. Thus, many contact (taste) receptors occur on the mouthparts, such as the labella of higher Diptera (Box 15.5) where salt and sugar receptors occur, and on the ovipositor, to assist with identification of suitable oviposition sites. The antennae, which often are forward-directed and prominent, are first to encounter sensory stimuli and are endowed with many distant chemoreceptors, some contact chemoreceptors, and many mechanoreceptors. The legs, particularly the tarsi which are in contact with the substrate, also have many chemoreceptors. In butterflies, stimulation of the tarsi by sugar solutions evokes an automatic extension of the proboscis. In blow flies, a complex sequence of stereotyped feeding behaviors is induced when a tarsal chemoreceptor is stimulated with sucrose. The proboscis starts to extend and, following sucrose stimulation of the chemoreceptors on the labellum, further proboscis extension occurs and the labellar lobes open. With more sugar stimulus, the source is sucked until stimulation of the mouthparts ceases. When this happens, a predictable pattern of search for further food follows.
Insect chemoreceptors are sensilla with one or more pores (holes). Two classes of sensilla can be defined based on their ultrastructure: uniporous, with one pore, and multiporous, with several to many pores. Uniporous sensilla range in appearance from hairs to pegs, plates, or simply pores in a cuticular depression, but all have relatively thick walls and a simple permeable pore, which may be apical or central. The hair or peg contains a chamber, which is in basal contact with a dendritic chamber that lies beneath the cuticle. The outer chamber may extrude a viscous liquid, presumed to assist in the entrapment and transfer of chemicals to the dendrites. It is assumed that these uniporous chemoreceptors predominantly detect chemicals by contact, although there is evidence for some olfactory function. Gustatory (contact) neurons are classified best according to their function and thus, in relation to feeding, there are cells whose activity in response to chemical stimulation either is to enhance or reduce feeding. These receptors are called phagostimulatory or deterrent.
The major olfactory role comes from multiporous sensilla, which are hair- or peg-like setae, with many round pores or slits in the thin walls, leading into a chamber known as the pore kettle. This is richly endowed with pore tubules, which run inwards to meet multibranched dendrites (Box 4.3). Development of an electroantennogram (Box 4.2) allowed revelation of the specificity of chemoreception by the antenna. Used in conjunction with the scanning electron microscope, micro-electrophysiology and modern molecular techniques have extended our understanding of the ability of insects to detect and respond to very weak chemical signals (Box 4.3). Great sensitivity is achieved by spreading very many receptors over as great an area as possible, and allowing the maximum volume of air to flow across the receptors. Thus, the antennae of many male moths are large and frequently the surface area is enlarged by pectinations that form a sieve-like basket (Fig. 4.6). Each antenna of the male silkworm moth (Bombycidae: Bombyx mori) has some 17,000 sensilla of different sizes and several ultrastructural morphologies. Sensilla respond specifically to sex-signaling chemicals produced by the female (sex pheromones; see below). As each sensillum has up to 3000 pores, each 10–15 nm in diameter, there are some 45 million pores per moth. Calculations concerning the silkworm moth suggest that just a few molecules could stimulate a nerve impulse above the background rate, and behavioral change may be elicited by less than a hundred molecules.
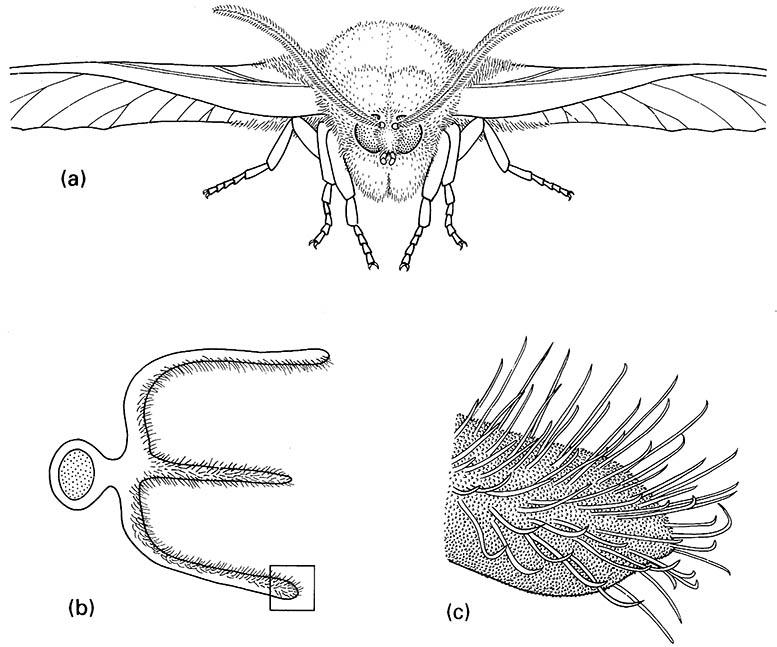
(a) anterior view of head showing tripectinate antennae of this species; (b) cross-section through the antenna showing the three branches; (c) enlargement of tip of outer branch of one pectination showing olfactory sensilla.

