4.1.3. Sound reception
Sound is a pressure fluctuation transmitted in a wave form via movement of the air or the substrate, including water. Sound and hearing are terms often applied to the quite limited range of frequencies of airborne vibration that humans perceive with their ears, usually in adults from 20 to 20,000 Hz (1 hertz (Hz) is a frequency of one cycle per second). Such a definition of sound is restrictive, particularly as amongst insects some receive vibrations ranging from as low as 1–2 Hz to ultrasound frequencies perhaps as high as 100 kHz. Specialized emission and reception across this range of frequencies of vibration are considered here. The reception of these frequencies involves a variety of organs, none of which resemble the ears of mammals.
An important role of insect sound is in intraspecific acoustic communication. For example, courtship in most orthopterans is acoustic, with males producing species-specific sounds (“songs”) that the predominantly non-singing females detect and upon which they base their choice of mate. Hearing also allows detection of predators, such as insectivorous bats, which use ultrasound in hunting. Probably each species of insect detects sound within one or two relatively narrow ranges of frequencies that relate to these functions.
The insect mechanoreceptive communication system can be viewed as a continuum from substrate vibration reception, grading through the reception of only very near airborne vibration to hearing of even quite distant sound using thin cuticular membranes called tympani (singular: tympanum; adjective: tympanal). Substrate signaling probably appeared first in insect evolution; the sensory organs used to detect substrate vibrations appear to have been co-opted and modified many times in different insect groups to allow reception of airborne sound at considerable distance and a range of frequencies.
Non-tympanal vibration reception
Two types of vibration or sound reception that do not involve tympani (see here) are the detection of substrate-borne signals and the ability to perceive the relatively large translational movements of the surrounding medium (air or water) that occur very close to a sound. The latter, referred to as near-field sound, is detected by either sensory hairs or specialized sensory organs.
A simple form of sound reception occurs in species that have very sensitive, elongate, trichoid sensilla that respond to vibrations produced by a near-field sound. For example, caterpillars of the noctuid moth Barathra brassicae have thoracic hairs about 0.5 mm long that respond optimally to vibrations of 150 Hz. Although in air this system is effective only for locally produced sounds, caterpillars can respond to the vibrations caused by audible approach of parasitic wasps.
The cerci of many insects, especially crickets, are clothed in long, fine trichoid sensilla (filiform setae or hairs) that are sensitive to air currents, which can convey information about the approach of predatory or parasitic insects or a potential mate. The direction of approach of another animal is indicated by which hairs are deflected; the sensory neuron of each hair is tuned to respond to movement in a particular direction. The dynamics (the time-varying pattern) of air movement gives information on the nature of the stimulus (and thus on what type of animal is approaching) and is indicated by the properties of the mechanosensory hairs. The length of each hair determines the response of its sensory neuron to the stimulus: neurons that innervate short hairs are most sensitive to high-intensity, high- frequency stimuli, whereas long hairs are more sensitive to low-intensity, low-frequency stimuli. The responses of many sensory neurons innervating different hairs on the cerci are integrated in the central nervous system to allow the insect to make a behaviorally appropriate response to detected air movement.
For low-frequency sounds in water (a medium more viscous than air), longer distance transmission is possible. Currently, however, rather few aquatic insects have been shown to communicate through underwater sounds. Notable examples are the “drumming” sounds that some aquatic larvae produce to assert territory, and the noises produced by underwater diving hemipterans such as corixids and nepids.
Many insects can detect vibrations transmitted through a substrate at a solid—air or solid—water boundary or along a water—air surface. The perception of substrate vibrations is particularly important for ground-dwelling insects, especially nocturnal species, and social insects living in dark nests. Some insects living on plant surfaces, such as sawflies (Hymenoptera: Pergidae), communicate with each other by tapping the stem. Various plant-feeding bugs (Hemiptera), such as leafhoppers, planthoppers, and pentatomids, produce vibratory signals that are transmitted through the host plant. Water-striders (Hemiptera: Gerridae), which live on the aquatic surface film, send pulsed waves across the water surface to communicate in courtship and aggression. Moreover, they can detect the vibrations produced by the struggles of prey that fall onto the water surface. Whirligig beetles (Gyrinidae; Fig. 10.8) can navigate using a form of echolocation: waves that move on the water surface ahead of them and are reflected from obstacles are sensed by their antennae in time to take evasive action.
The specialized sensory organs that receive vibrations are subcuticular mechanoreceptors called chordotonal organs. An organ consists of one to many scolopidia, each of which consists of three linearly arranged cells: a subtympanal cap cell placed on top of a sheath cell (scolopale cell), which envelops the end of a nerve cell dendrite (Fig. 4.3). All adult insects and many larvae have a particular chordotonal organ, Johnston’s organ, lying within the pedicel, the second antennal segment. The primary function is to sense movements of the antennal flagellum relative to the rest of the body, as in detection of flight speed by air movement. Additionally, it functions in hearing in some insects. In male mosquitoes (Culicidae) and midges (Chironomidae), many scolopidia are contained in the swollen pedicel. These scolopidia are attached at one end to the pedicel wall and at the other, sensory end to the base of the third antennal segment. This greatly modified Johnston’s organ is the male receptor for the female wing tone (see section 4.1.4), as shown when males are rendered unreceptive to the sound of the female by amputation of the terminal flagellum or arista of the antenna.
Detection of substrate vibration involves the subgenual organ, a chordotonal organ located in the proximal tibia of each leg. Subgenual organs are found in most insects except the Coleoptera and Diptera. The organ consists of a semicircle of many sensory cells lying in the hemocoel, connected at one end to the inner cuticle of the tibia, and at the other to the trachea. There are subgenual organs within all legs: the organs of each pair of legs may respond specifically to substrate-borne sounds of differing frequencies. Vibration reception may involve either direct transfer of low- frequency substrate vibrations to the legs, or there may be more complex amplification and transfer. Airborne vibrations can be detected if they cause vibration of the substrate and hence of the legs.
Tympanal reception
The most elaborate sound reception system in insects involves a specific receptor structure, the tympanum. This membrane responds to distant sounds transmitted by airborne vibration. Tympanal membranes are linked to chordotonal organs and are associated with air-filled sacs, such as modifications of the trachea, that enhance sound reception. Tympanal organs are located on the:
- ventral thorax between the metathoracic legs of mantids;
- metathorax of many noctuid moths;
- prothoracic legs of many orthopterans;
- abdomen of other orthopterans, cicadas, and some moths and beetles;
- wing bases of certain moths and lacewings;
- prosternum of some flies (Box 4.1);
- cervical membranes of a few scarab beetles.
The differing location of these organs and their occurrence in distantly related insect groups indicates that tympanal hearing evolved several times in insects. Neuroanatomical studies suggest that all insect tympanal organs evolved from proprioceptors, and the wide distribution of proprioceptors throughout the insect cuticle must account for the variety of positions of tympanal organs.
Tympanal sound reception is particularly well developed in orthopterans, notably in the crickets and katydids. In most of these ensiferan Orthoptera the tympanal organs are on the tibia of each fore leg (Figs. 4.4 & 9.2a). Behind the paired tympanal membranes lies an acoustic trachea that runs from a pro- thoracic spiracle down each leg to the tympanal organ (Fig. 4.4a).
Crickets and katydids have similar hearing systems. The system in crickets appears to be less specialized because their acoustic tracheae remain connected to the ventilatory spiracles of the prothorax. The acoustic tracheae of katydids form a system completely isolated from the ventilatory tracheae, opening via a separate pair of acoustic spiracles. In many katydids, the tibial base has two separated longitudinal slits each of which leads into a tympanic chamber (Fig. 4.4b). The acoustic trachea, which lies centrally in the leg, is divided in half at this point by a membrane, such that one half closely connects with the anterior and the other half with the posterior tympanal membrane. The primary route of sound to the tympanal organ is usually from the acoustic spiracle and along the acoustic trachea to the tibia. The change in cross-sectional area from the enlargement of the trachea behind each spiracle (some- times called a tracheal vesicle) to the tympanal organ in the tibia approximates the function of a horn and amplifies the sound. Although the slits of the tympanic chambers do allow the entry of sound, their exact function is debatable. They may allow directional hearing, because very small differences in the time of arrival of sound waves at the tympanum can be detected by pressure differences across the membrane.
Whatever the major route of sound entry to the tympanal organs, air- and substrate-borne acoustic signals cause the tympanal membranes to vibrate. Vibrations are sensed by three chordotonal organs: the subgenual organ, the intermediate organ, and the crista acustica (Fig. 4.4c). The subgenual organs, which have a form and function like those of non-orthopteroid insects, are present on all legs but the crista acustica and intermediate organs are found only on the fore legs in conjunction with the tympana. This implies that the tibial hearing organ is a serial homologue of the proprioceptor units of the mid and hind legs.
The crista acustica consists of a row of up to 60 scolopidial cells attached to the acoustic trachea and is the main sensory organ for airborne sound in the 5–50 kHz range. The intermediate organ, which consists of 10–20 scolopidial cells, is posterior to the subgenual organ and virtually continuous with the crista acustica. The role of the intermediate organ is uncertain but it may respond to airborne sound of frequencies from 2 to 14 kHz. Each of the three chordotonal organs is innervated separately, but the neuronal connections between the three imply that signals from the different receptors are integrated.
Hearing insects can identify the direction of a point source of sound, but exactly how they do so varies between taxa. Localization of sound directionality clearly depends upon detection of differences in the sound received by one tympanum relative to another, or in some orthopterans by a tympanum within a single leg. Sound reception varies with the orientation of the body relative to the sound source, allowing some precision in locating the source. The unusual means of sound reception and sensitivity of detection of direction of sound source shown by ormiine flies is discussed in Box 4.1.
Night activity is common, as shown by the abundance and diversity of insects attracted to artificial light, especially at the ultraviolet end of the spectrum, and on moonless nights. Night flight allows avoidance of visual-hunting predators, but exposes the insect to specialist nocturnal predators — the insectivorous bats (Microchiroptera). These bats employ a biological sonar system using ultrasonic frequencies that range (according to species) from 20 to 200 kHz for navigating and for detecting and locating prey, predominantly flying insects.
Although bat predation on insects occurs in the darkness of night and high above a human observer, it is evident that a range of insect taxa can detect bat ultrasounds and take appropriate evasive action. The behavioral response to ultrasound, called the acoustic startle response, involves very rapid and co-ordinated muscle contractions. This leads to reactions such as “freezing”, unpredictable deviation in flight, or rapid cessation of flight and plummeting towards the ground. Instigation of these reactions, which assist in escape from predation, obviously requires that the insect hears the ultrasound produced by the bat. Physiological experiments show that within a few milliseconds of the emission of such a sound the response takes place, which would precede the detection of the prey by a bat.
To date, insects belonging to five orders have been shown to be able to detect and respond to ultrasound: lacewings (Neuroptera), beetles (Coleoptera), praying mantids (Mantodea), moths (Lepidoptera), and locusts, katydids, and crickets (Orthoptera). Tympanal organs occur in different sites amongst these insects, showing that ultrasound reception has several independent origins amongst these insects. As seen earlier in this chapter (here), the Orthoptera are major acoustic communicators that use sound in intraspecific sexual signaling. Evidently, hearing ability arose early in orthopteran evolution, probably at least some 200 mya, long before bats evolved (perhaps a little before the Eocene (50 mya) from which the oldest fossil comes). Thus, orthopteran ability to hear bat ultrasounds can be seen as an exaptation — a morphological—physiological predisposition that has been modified to add sensitivity to ultrasound. The crickets, bush- crickets, and acridid grasshoppers that communicate intraspecifically and also hear ultrasound have sensitivity to high- and low-frequency sound — and perhaps limit their discrimination to only two discrete frequencies. The ultrasound elicits aversion; the other (under suitable conditions) elicits attraction.
In contrast, the tympanal hearing that has arisen independently in several other insects appears to be receptive specifically to ultrasound. The two receptors of a “hearing” noctuoid moth, though differing in threshold, are tuned to the same ultrasonic frequency, and it has been demonstrated experimentally that the moths show behavioral (startle) and physiological (neural) response to bat sonic frequencies. In the parasitic tachinid fly Ormia (Box 4.1), in which the female fly locates its orthopteran host by tracking its mating calls, the structure and function of the “ear” is sexually dimorphic. The tympanic area of the female fly is larger, and is sensitive to the 5 kHz frequency of the cricket host and also to the 20–60 kHz ultrasounds made by insectivorous bats, whereas the smaller tympanic area of the male fly responds only to the ultrasound. This suggests that the acoustic response originally was present in both sexes and was used to detect and avoid bats, with sensitivity to cricket calls a later modification in the female sex alone.
At least in these cases, and probably in other groups in which tympanal hearing is limited in taxonomic range and complexity, ultrasound reception appears to have coevolved with the sonic production of the bats that seek to eat them.
Note: the divided compound eye allows the beetle to see both above and below water simultaneously; hydrofuge hairs on the margin of the elytra repel water. (After White et al. 1984)
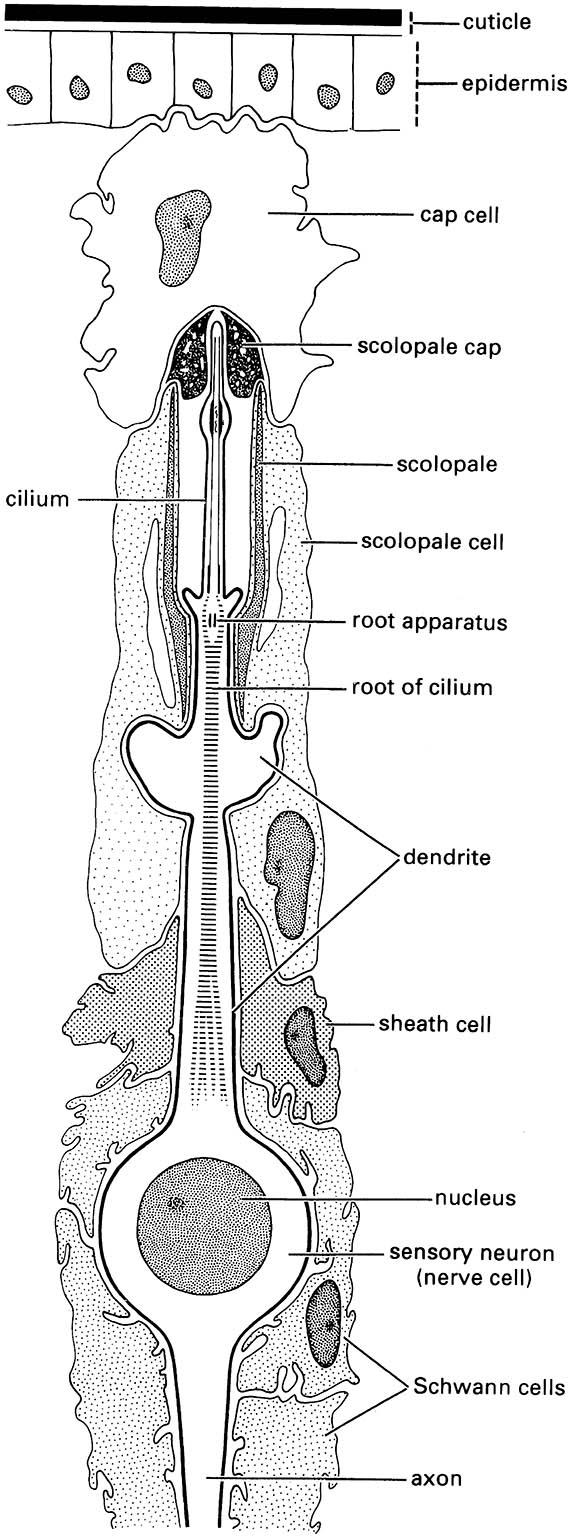
(After Gray 1960)
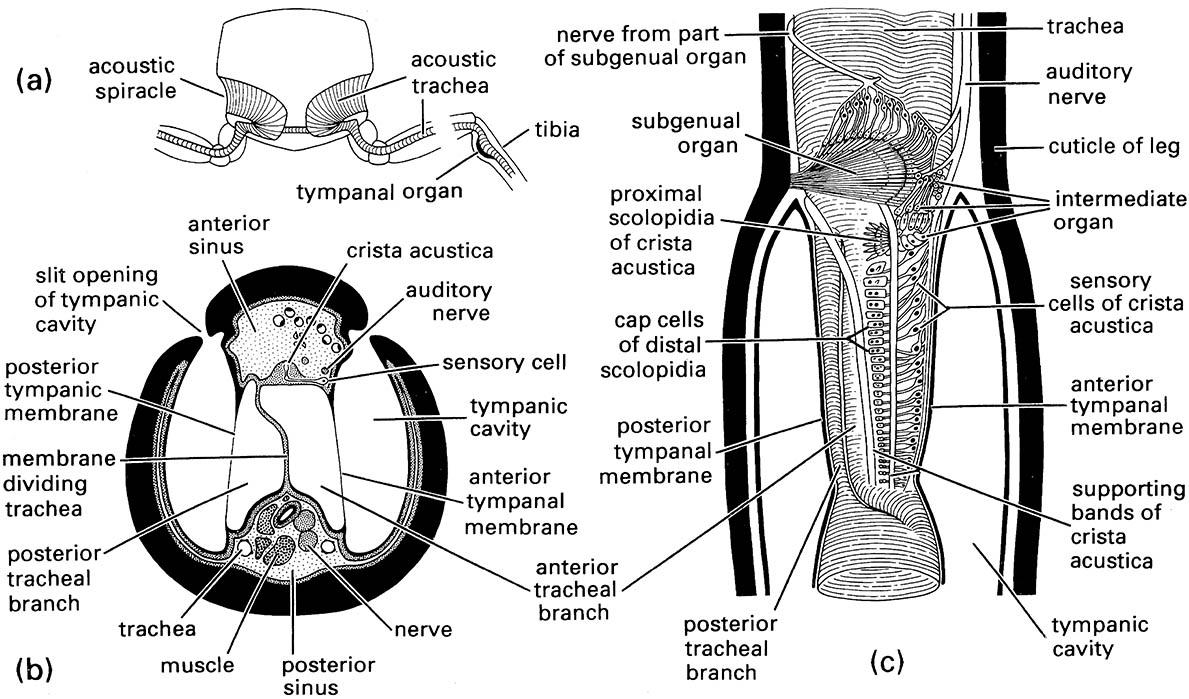
(a) transverse section through the fore legs and prothorax to show the acoustic spiracles and tracheae; (b) transverse section through the base of the fore tibia; (c) longitudinal breakaway view of the fore tibia. (After Schwabe 1906; in Michelsen & Larsen 1985)
(a) a mole cricket of Gryllotalpa (Orthoptera: Gryllotalpidae); (b) a nymphal periodical cicada of Magicicada (Hemiptera: Cicadidae); and (c) a scarab beetle of Canthon (Coleoptera: Scarabaeidae). ((a) After Frost 1959; (b) after Snodgrass 1967; (c) after Richards & Davies 1977)

