11.2.4. Gall induction
Insect-induced plant galls result from a very specialized type of insect—plant interaction in which the morphology of plant parts is altered, often substantially and characteristically, by the influence of the insect. Generally, galls are defined as pathologically developed cells, tissues, or organs of plants that have arisen by hypertrophy (increase in cell size) and/or hyperplasia (increase in cell number) as a result of stimulation from foreign organisms. Some galls are induced by viruses, bacteria, fungi, nematodes, and mites, but insects cause many more. The study of plant galls is called cecidology, gall-causing animals (insects, mites, and nematodes) are cecidozoa, and galls induced by cecidozoa are referred to as zoocecidia. Cecidogenic insects account for about 2% of all described insect species, with perhaps 13,000 species known. Although galling is a worldwide phenomenon across most plant groups, global survey shows an eco-geographical pattern with gall incidence more frequent in vegetation with a sclerophyllous habit, or at least living on plants in wet—dry seasonal environments.
On a world basis, the principal cecidozoa in terms of number of species are representatives of just three orders of insects — the Hemiptera, Diptera, and Hymenoptera. In addition, about 300 species of mostly tropical Thysanoptera (thrips) are associated with galls, although not necessarily as inducers, and some species of Coleoptera (mostly weevils) and microlepidoptera (small moths) induce galls. Most hemipteran galls are elicited by Sternorrhyncha, in particular aphids, coccoids, and psyllids; their galls are structurally diverse and those of gall-inducing eriococcids (Coccoidea: Eriococcidae) often exhibit spectacular sexual dimorphism, with galls of female insects much larger and more complex than those of their conspecific males (Fig. 11.5a,b). Worldwide, there are several hundred gall-inducing coccoid species in about 10 families, about 350 gall-forming Psylloidea, mostly in two families, and perhaps 700 gall-inducing aphid species distributed among the three families, Phylloxeridae (Box 11.2), Adelgidae, and Aphididae.
The Diptera contains the highest number of gall- inducing species, perhaps thousands, but the probable number is uncertain because many dipteran gall inducers are poorly known taxonomically. Most cecidogenic flies belong to one family of at least 4500 species, the Cecidomyiidae (gall midges), and induce simple or complex galls on leaves, stems, flowers, buds, and even roots. The other fly family that includes some important cecidogenic species is the Tephritidae, in which gall inducers mostly affect plant buds, often of the Asteraceae. Galling species of both cecidomyiids and tephritids are of actual or potential use for biological control of some weeds. Three superfamilies of wasps contain large numbers of gall-inducing species: Cynipoidea contains the gall wasps (Cynipidae, at least 1300 species), which are among the best-known gall insects in Europe and North America, where hundreds of species form often extremely complex galls, especially on oaks and roses (Fig. 11.5c,d); Tenthredinoidea has a number of gall-forming sawflies, such as Pontania species (Tethredinidae) (Fig. 11.5g); and Chalcidoidea includes several families of gall inducers, especially species in the Agaonidae (fig wasps; Box 11.4), Eurytomidae, and Pteromalidae.
There is enormous diversity in the patterns of development, shape, and cellular complexity of insect galls (Fig. 11.5). They range from relatively undifferentiated masses of cells (“indeterminate” galls) to highly organized structures with distinct tissue layers (“determinate” galls). Determinate galls usually have a shape that is specific to each insect species. Cynipids, cecidomyiids, and eriococcids form some of the most histologically complex and specialized galls; these galls have distinct tissue layers or types that may bear little resemblance to the plant part from which they are derived. Among the determinate galls, different shapes correlate with mode of gall formation, which is related to the initial position and feeding method of the insect (as discussed below). Some common types of galls are:
- covering galls, in which the insect becomes enclosed within the gall, either with an opening (ostiole) to the exterior, as in coccoid galls (Fig. 11.5a,b), or without any ostiole, as in cynipid galls (Fig. 11.5c);
- filz galls, which are characterized by their hairy epidermal outgrowths (Fig. 11.5d);
- roll and fold galls, in which differential growth provoked by insect feeding results in rolled or twisted leaves, shoots, or stems, which are often swollen, as in many aphid galls (Fig. 11.5e);
- pouch galls, which develop as a bulge of the leaf blade, forming an invaginated pouch on one side and a prominent bulge on the other, as in many psyllid galls (Fig. 11.5f );
- mark galls, in which the insect egg is deposited inside stems or leaves so that the larva is completely enclosed throughout its development, as in sawfly galls (Fig. 11.5g);
- pit galls, in which a slight depression, sometimes surrounded by a swelling, is formed where the insect feeds;
- bud and rosette galls, which vary in complexity and cause enlargement of the bud or sometimes multiplication and miniaturization of new leaves, forming a pine-cone-like gall.
Gall formation may involve two separate processes: (i) initiation and (ii) subsequent growth and maintenance of structure.
Usually, galls can be stimulated to develop only from actively growing plant tissue. Therefore, galls are initiated on young leaves, flower buds, stems, and roots, and rarely on mature plant parts. Some complex galls develop only from undifferentiated meristematic tissue, which becomes molded into a distinctive gall by the activities of the insect. Development and growth of insect-induced galls (including, if present, the nutritive cells upon which some insects feed) depend upon continued stimulation of the plant cells by the insect. Gall growth ceases if the insect dies or reaches maturity. It appears that gall insects, rather than the plants, control most aspects of gall formation, largely via their feeding activities.
The mode of feeding differs in different taxa as a con- sequence of fundamental differences in mouthpart structure. The larvae of gall-inducing beetles, moths, and wasps have biting and chewing mouthparts, whereas larval gall midges and nymphal aphids, coccoids, psyllids, and thrips have piercing and sucking mouthparts. Larval gall midges have vestigial mouthparts and largely absorb nourishment by suction. Thus, these different insects mechanically damage and deliver chemicals (or perhaps genetic material, see below) to the plant cells in a variety of ways.
Little is known about what stimulates gall induction and growth. Wounding and plant hormones (such as cytokinins) appear important in indeterminate galls, but the stimuli are probably more complex for determinate galls. Oral secretions, anal excreta, and accessory gland secretions have been implicated in different insect—plant interactions that result in determinate galls. The best-studied compounds are the salivary secretions of Hemiptera. Salivary substances, including amino acids, auxins (and other plant growth regulators), phenolic compounds, and phenol oxidases, in various concentrations, may have a role either in gall initiation and growth or in overcoming the defensive necrotic reactions of the plant. Plant hormones, such as auxins and cytokinins, must be involved in cecido-genesis but it is equivocal whether these hormones are produced by the insect, by the plant as a directed response to the insect, or are incidental to gall induction. In certain complex galls, such as those of eriococcoids and cynipids, it is conceivable that the development of the plant cells is redirected by semiautonomous genetic entities (viruses, plasmids, or transposons) transferred from the insect to the plant. Thus, the initiation of such galls may involve the insect acting as a DNA or RNA donor, as in some wasps that parasitize insect hosts (Box 13.1). Unfortunately, in comparison with anatomical and physiological studies of galls, genetic investigations are in their infancy.
The gall-inducing habit may have evolved either from plant mining and boring (especially likely for Lepidoptera, Hymenoptera, and certain Diptera) or from sedentary surface feeding (as is likely for Hemiptera, Thysanoptera, and cecidomyiid Diptera). It is believed to be beneficial to the insects, rather than a defensive response of the plant to insect attack. All gall insects derive their food from the tissues of the gall and also some shelter or protection from natural enemies and adverse conditions of temperature or moisture. The relative importance of these environmental factors to the origin of the galling habit is difficult to ascertain because current advantages of gall living may differ from those gained in the early stages of gall evolution. Clearly, most galls are “sinks” for plant assimilates — the nutritive cells that line the cavity of wasp and fly galls contain higher concentrations of sugars, protein, and lipids than ungalled plant cells. Thus, one advantage of feeding on gall rather than normal plant tissue is the availability of high-quality food. Moreover, for sedentary surface feeders, such as aphids, psyllids, and coccoids, galls furnish a more protected microenvironment than the normal plant surface. Some cecidozoa may “escape” from certain parasitoids and predators that are unable to penetrate galls, particularly galls with thick woody walls.
Other natural enemies, however, specialize in feeding on gall-living insects or their galls and sometimes it is difficult to determine which insects were the original inhabitants. Some galls are remarkable for the association of an extremely complex community of species, other than the gall causer, belonging to diverse insect groups. These other species may be either parasitoids of the gall former (i.e. parasites that cause the eventual death of their host; Chapter 13) or inquilines (“guests” of the gall former) that obtain their nourishment from tissues of the gall. In some cases, gall inquilines cause the original inhabitant to die through abnormal growth of the gall; this may obliterate the cavity in which the gall former lives or prevent emergence from the gall. If two species are obtained from a single gall or a single type of gall, one of these insects must be a parasitoid, an inquiline, or both. There are even cases of hyperparasitism, in which the parasitoids themselves are subject to parasitization (section 13.3.1).
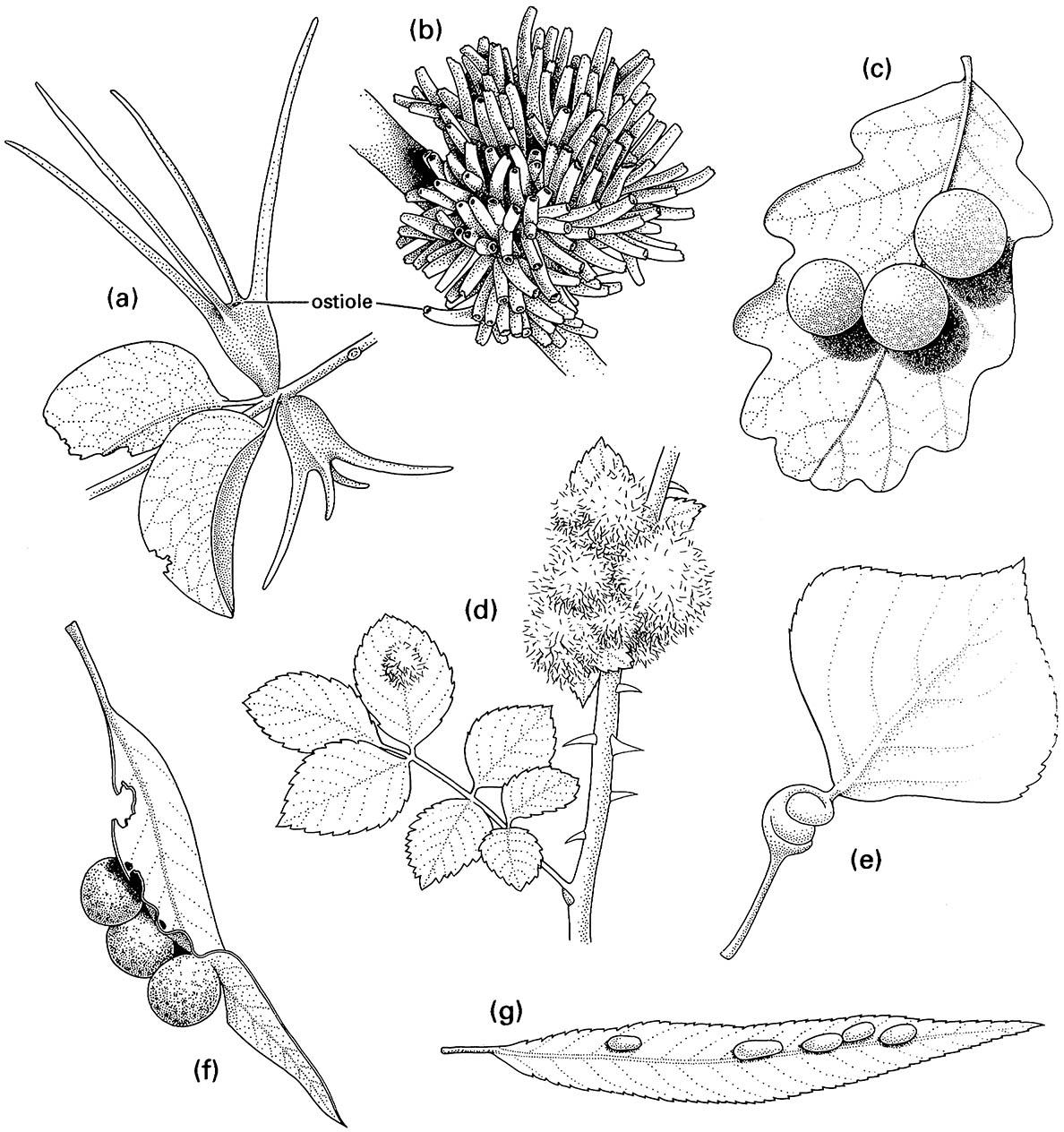
(a) two coccoid galls, each formed by a female of Apiomorpha munita (Hemiptera: Eriococcidae) on the stem of Eucalyptus melliodora; (b) a cluster of galls each containing a male of A. munita on E. melliodora; (c) three oak cynipid galls formed by Cynips quercusfolii (Hymenoptera: Cynipidae) on a leaf of Quercus sp.; (d) rose bedeguar galls formed by Diplolepis rosae (Hymenoptera: Cynipidae) on Rosa sp.; (e) a leaf petiole of lombardy poplar, Populus nigra, galled by the aphid Pemphigus spirothecae (Hemiptera: Aphididae); (f) three psyllid galls, each formed by a nymph of Glycaspis sp. (Hemiptera: Psyllidae) on a eucalypt leaf; (g) willow bean galls of the sawfly Pontania proxima (Hymenoptera: Tenthredinidae) on a leaf of Salix sp. ((d-g ) After Darlington 1975)

