11. Insects and plants
Insects and plants share ancient associations that date from the Carboniferous, some 300 million years ago (Fig. 8.1). Evidence preserved in fossilized plant parts of insect damage indicates a diversity of types of phytophagy (plant-feeding) by insects, which are presumed to have had different mouthparts, and associated with tree and seed ferns from Late Carboniferous coal deposits. Prior to the origin of the now dominant angiosperms (flowering plants), the diversification of other seed-plants, namely conifers, seed ferns, cycads, and (extinct) bennettiales, provided the template for radiation of insects with specific plant-feeding associations. Some of these, such as weevils and thrips with cycads, persist to this day. However, the major diversification of insects became manifest later, in the Cretaceous period. At this time, angiosperms dramatically increased in diversity in a radiation that displaced the previously dominant plant groups of the Jurassic period. Interpreting the early evolution of the angiosperms is contentious, partly because of the paucity of fossilized flowers prior to the period of radiation, and also because of the apparent rapidity of the origin and diversification within the major angiosperm families.
However, according to estimates of their phylogeny, the earliest angiosperms may have been insect-pollinated, perhaps by beetles. Many living representatives of primitive families of beetles feed on fungi, fern spores, or pollen of other non-angiosperm taxa such as cycads. As this feeding type preceded the angiosperm radiation, it can be seen as a preadaptation for angiosperm pollination. The ability of flying insects to transport pollen from flower to flower on different plants is fundamental to cross-pollination. Other than the beetles, the most significant and diverse present-day pollinator taxa belong to three orders — the Diptera (flies), Hymenoptera (wasps and bees), and Lepidoptera (moths and butterflies). Pollinator taxa within these orders are unrepresented in the fossil record until late in the Cretaceous. Although insects almost certainly pollinated cycads and other primitive plants, insect pollinators may have promoted speciation in angiosperms, through pollinator-mediated isolating mechanisms.
As seen in Chapter 9, many modern-day non-insect hexapods and apterygote insects scavenge in soil and litter, predominantly feeding on decaying plant material. The earliest true insects probably fed similarly. This manner of feeding certainly brings soil-dwelling insects into contact with plant roots and subterranean storage organs, but specialized use of plant aerial parts by sap sucking, leaf chewing, and other forms of phytophagy arose later in the phylogeny of the insects. Feeding on living tissues of higher plants presents problems that are experienced neither by the scavengers living in the soil or litter, nor by predators. First, to feed on leaves, stems, or flowers a phytophagous insect must be able to gain and retain a hold on the vegetation. Second, the exposed phytophage may be subject to greater desiccation than an aquatic or litter-dwelling insect. Third, a diet of plant tissues (excluding seeds) is nutritionally inferior in protein, sterol, and vitamin content compared with food of animal or microbial origin. Last, but not least, plants are not passive victims of phytophages, but have evolved a variety of means to deter herbivores. These include physical defenses, such as spines, spicules or sclerophyllous tissue, and/or chemical defenses that may repel, poison, reduce food digestibility, or otherwise adversely affect insect behavior and/or physiology. Despite these barriers, about half of all living insect species are phytophagous, and the exclusively plant-feeding Lepidoptera, Curculionidae (weevils), Chrysomelidae (leaf beetles), Agromyzidae (leaf-mining flies), and Cynipidae (gall wasps) are very speciose. Plants represent an abundant resource and insect taxa that can exploit this have flourished in association with plant diversification (section 1.3.4).
This chapter begins with a consideration of the evolutionary interactions among insects and their plant hosts, amongst which a euglossine bee pollinator at work on the flower of a Stanhopea orchid, a chrysomelid beetle feeding on the orchid leaf, and a pollinating bee fly hovering nearby are illustrated in the chapter vignette. The vast array of interactions of insects and living plants can be grouped into three categories, defined by the effects of the insects on the plants. Phytophagy (herbivory) includes leaf chewing, sap sucking, seed predation, gall induction, and mining the living tissues of plants (section 11.2). The second category of interactions is important to plant reproduction and involves mobile insects that transport pollen between conspecific plants (pollination) or seeds to suitable germination sites (myrmecochory). These interactions are mutualistic because the insects obtain food or some other resource from the plants that they service (section 11.3). The third category of insect—plant interaction involves insects that live in specialized plant structures and provide their host with either nutrition or defense against herbivores, or both (section 11.4). Such mutualisms, like the nutrient-producing fly larvae that live unharmed within the pitchers of carnivorous plants, are unusual but provide fascinating opportunities for evolutionary and ecological studies. There is a vast literature dealing with insect—plant interactions and the interested reader should consult the reading list at the end of this chapter.
The chapter concludes with seven taxonomic boxes that summarize the morphology and biology of the primarily phytophagous orders Orthoptera, Phasmatodea, Thysanoptera, Hemiptera, Psocoptera, Coleoptera, and Lepidoptera.
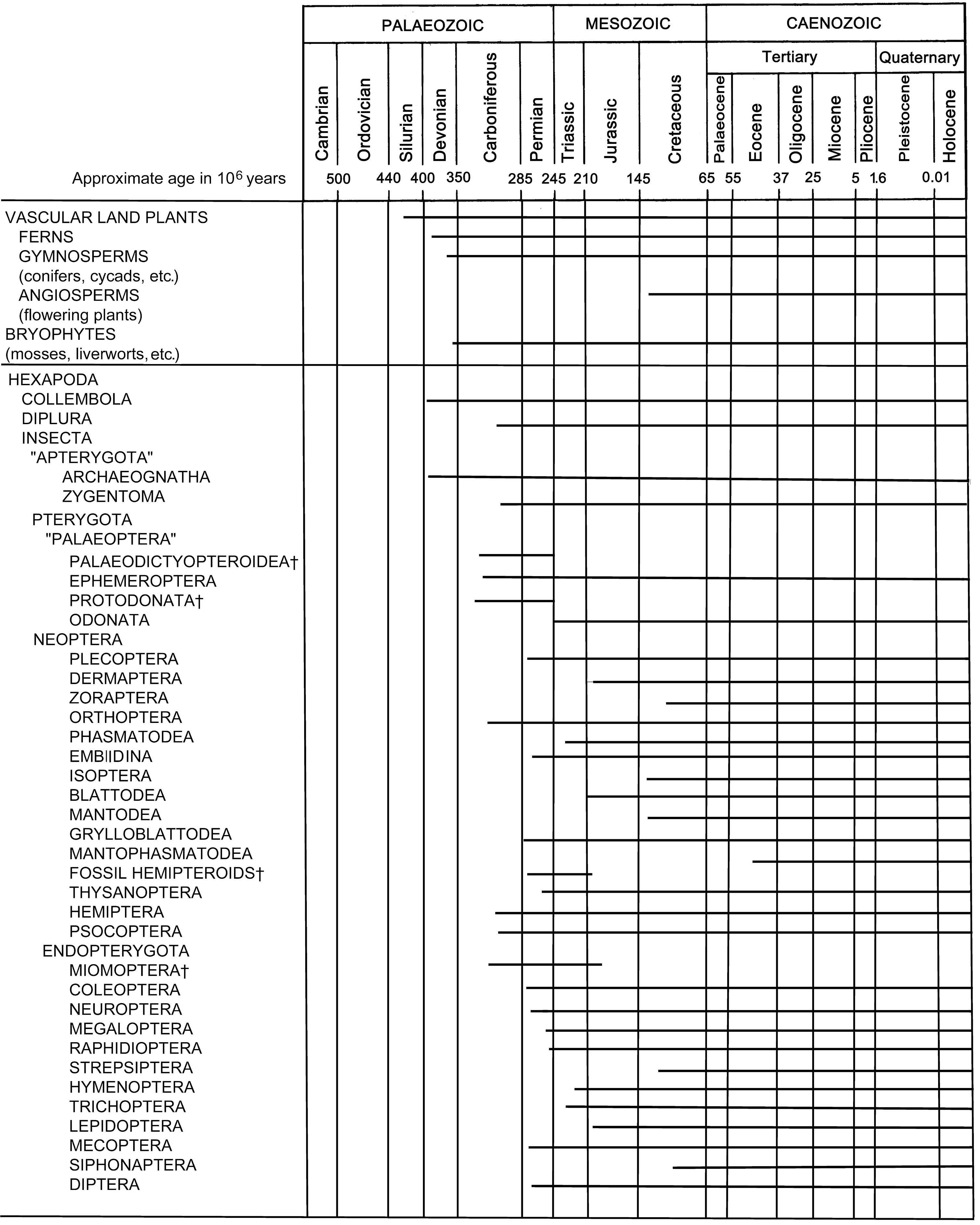
Taxa that contain only fossils are indicated by the symbol †. The fossil record of Protura, Phthiraptera, and Zoraptera dates from only the Holocene, presumably because of the sma ll size and/or rarity of these organisms. Miomoptera represent the earliest fossil endopterygotes. The placement of Rhyniognatha is unknown. (Insect records after Kukalová-Peck 1991; and Conrad Labandeira (Smithsonian Institution) pers. comm.)

