11.3.1. Pollination
Sexual reproduction in plants involves pollination — the transfer of pollen (male germ cells in a protective covering) from the anthers of a flower to the stigma (Fig. 11.7a). A pollen tube grows from the stigma down the style to an ovule in the ovary where it fertilizes the egg. Pollen generally is transferred either by an animal pollinator or by the wind. Transfer may be from anthers to stigma of the same plant (either of the same flower or a different flower) (self-pollination), or between flowers on different plants (with different genotypes) of the same species (cross-pollination). Animals, especially insects, pollinate most flowering plants. It is argued that the success of the angiosperms relates to the development of these interactions. The benefits of insect pollination (entomophily) over wind pollination (anemophily) include:
- increase in pollination efficiency, including reduc- tion of pollen wastage;
- successful pollination under conditions unsuitable for wind pollination;
- maximization of the number of plant species in a given area (as even rare plants can receive conspecific pollen carried into the area by insects). Within-flower self-pollination also brings some of these advantages, but continued selfing induces deleterious homozygosity, and rarely is a dominant fertilization mechanism.
Generally, it is advantageous to a plant for its pollinators to be specialist visitors that faithfully pollinate only flowers of one or a few plant species. Pollinator constancy, which may initiate the isolation of small plant populations, is especially prevalent in the Orchidaceae — the most speciose family of vascular plants.
The major anthophilous (flower-frequenting) taxa among insects are the beetles (Coleoptera), flies (Diptera), wasps, bees, and ants (Hymenoptera), thrips (Thysanoptera), and butterflies and moths (Lepidoptera). These insects visit flowers primarily to obtain nectar and/or pollen, but even some predatory insects may pollinate the flowers that they visit. Nectar primarily consists of a solution of sugars, especially glucose, fructose, and sucrose. Pollen often has a high protein content plus sugar, starch, fat, and traces of vitamins and inorganic salts. In the case of a few bizarre inter- actions, male hymenopterans are attracted neither by pollen nor by nectar but by the resemblance of certain orchid flowers in shape, color, and odor to their conspecific females (see Plate 4.4, facing here). In attempting to mate (pseudocopulate) with the insect-mimicking flower (see Plate 4.5), the male inadvertently pollinates the orchid with pollen that adhered to his body during previous pseudocopulations. Pseudo-copulatory pollination is common among Australian thynnine wasps (Tiphiidae), but occurs in a few other wasp groups, some bees, and rarely in ants.
Cantharophily (beetle pollination) may be the oldest form of insect pollination. Beetle-pollinated flowers often are white or dull colored, strong smelling, and regularly bowl- or dish-shaped (Fig. 11.7). Beetles mostly visit flowers for pollen, although nutritive tissue or easily accessible nectar may be utilized, and the plant’s ovaries usually are well protected from the biting mouthparts of their pollinators. The major beetle families that commonly or exclusively contain anthophilous species are the Buprestidae (jewel beetles; Fig. 11.7b), Cantharidae (soldier beetles), Cerambycidae (longicorn or longhorn beetles), Cleridae (checkered beetles), Dermestidae, Lycidae (net-winged beetles), Melyridae (soft-winged flower beetles), Mordellidae (tumbling flower beetles), Nitidulidae (sap beetles), and Scarabaeidae (scarabs).
Myophily (fly pollination) occurs when flies visit flowers to obtain nectar (see Plate 4.6), although hover flies (Syrphidae) feed chiefly on pollen rather than nectar. Fly-pollinated flowers tend to be less showy than other insect-pollinated flowers but may have a strong smell, often malodorous. Flies generally utilize many different sources of food and thus their pollinating activity is irregular and unreliable. However, their sheer abundance and the presence of some flies throughout the year mean that they are important pollinators for many plants. Both dipteran groups contain anthophilous species. Among the Nematocera, mosquitoes and bibionids are frequent blossom visitors, and predatory midges, principally of Forcipomyia species (Ceratopogonidae), are essential pollinators of cocoa flowers. Pollinators are more numerous in the Brachycera, in which at least 30 families are known to contain anthophilous species. Major pollinator taxa are the Bombyliidae (bee flies), Syrphidae, and muscoid families.
Many members of the large order Hymenoptera visit flowers for nectar and/or pollen. The Apocrita, which contains most of the wasps (as well as bees and ants), is more important than the Symphyta (sawflies) in terms of sphecophily (wasp pollination). Many pollinators are found in the superfamilies Ichneumonoidea and Vespoidea. Fig wasps (Chalcidoidea: Agaonidae) are highly specialized pollinators of the hundreds of species of figs (discussed in Box 11.4). Ants (Vespoidea: Formicidae) are rather poor pollinators, although myrmecophily (ant pollination) is known for a few plant species. Ants are commonly anthophilous (flower loving), but rarely pollinate the plants that they visit. Two hypotheses, perhaps acting together, have been postulated to explain the paucity of ant pollination. First, ants are flightless, often small, and their bodies frequently are smooth, thus they are unlikely to facilitate cross-pollination because the foraging of each worker is confined to one plant, they often avoid contact with the anthers and stigmas, and pollen does not adhere easily to them. Second, the metapleural glands of ants produce secretions that spread over the integument and inhibit fungi and bacteria, but also can affect pollen viability and germination. Some plants actually have evolved mechanisms to deter ants; however, a few, especially in hot dry habitats, appear to have evolved adaptations to ant pollination.
Generally, bees are regarded as the most important group of insect pollinators. They collect nectar and pollen for their brood as well as for their own consumption. There are over 20,000 species of bees worldwide and all are anthophilous. Plants that depend on melittophily (bee pollination) often have bright (yellow or blue), sweet-smelling flowers with nectar guides — lines (often visible only as ultraviolet light) on the petals that direct pollinators to the nectar. The main bee pollinator worldwide is the honey bee, Apis mellifera (Apidae). The pollination services provided by this bee are extremely important for many crop plants (section 1.2), but in natural ecosystems serious problems can be caused. Honey bees compete with native insect pollinators by depleting nectar and pollen supplies and may disrupt pollination by displacing the specialist pollinators of native plant species.
Most members of the Lepidoptera feed from flowers using a long, thin proboscis. In the speciose Ditrysia (the “higher” Lepidoptera) the proboscis is retractile (Fig. 2.12), allowing feeding and drinking from sources distant from the head. Such a structural innovation may have contributed to the radiation of this successful group, which contains 98% of all lepidopteran species. Flowers pollinated by butterflies and moths often are regular, tubular, and sweet smelling. Phalaenophily (moth pollination) typically is associated with light- colored, pendant flowers that have nocturnal or crepuscular anthesis (opening of flowers); whereas psychophily (butterfly pollination) is typified by red, yellow, or blue upright flowers that have diurnal anthesis.
Insect—plant interactions associated with pollination are clearly mutualistic. The plant is fertilized by appropriate pollen, and the insect obtains food (or sometimes fragrances) supplied by the plant, often specifically to attract the pollinator. It is clear that plants may experience strong selection as a result of insects. In contrast, in most pollination systems, evolution of the pollinators may have been little affected by the plants that they visited. For most insects, any particular plant is just another source of nectar or pollen and even insects that appear to be faithful pollinators over a short observation period may utilize a range of plants in their lifetime. Nevertheless, symmetrical influences do occur in some insect—plant pollination systems, as evidenced by the specializations of each fig wasp species to the fig species that it pollinates (Box 11.4), and by correlations between moth proboscis (tongue) lengths and flower depths for a range of orchids and some other plants. For example, the Malagasy star orchid, Angraecum sesquipedale, has floral spurs that may exceed 30 cm in length, and has a pollinator with a tongue length of some 22 cm, a giant hawkmoth, Xanthopan morgani praedicta (Sphingidae) (Fig. 11.8). Only this moth can reach the nectar at the apex of the floral spurs and, during the process of pushing its head into the flower, it pollinates the orchid. This is cited often as a spectacular example of a coevolved “long-tongued” pollinator, whose existence had been predicted by Charles Darwin and Alfred Russel Wallace, who knew of the long- spurred flower but not the hawkmoth. However, the interpretation of this relationship as coevolution has been challenged with the suggestion that the long tongue evolved in the nectar-feeding moth to evade (by distance-keeping and feeding in hovering flight) ambushing predators (e.g. spiders) lurking in other less-specialized flowers frequented by X. morgani. In this interpretation, pollination of A. sesquipedale follows a host-shift of the preadapted pollinator, with only the orchid showing adaptive evolution. The specificity of location of pollinia (pollen masses) on the tongue of X. morgani seems to argue against the pollinator-shift hypothesis, but detailed field study is required to resolve the controversy. Unfortunately, this rare Malagasy insect—plant system is threatened because its natural rainforest habitat is being destroyed.
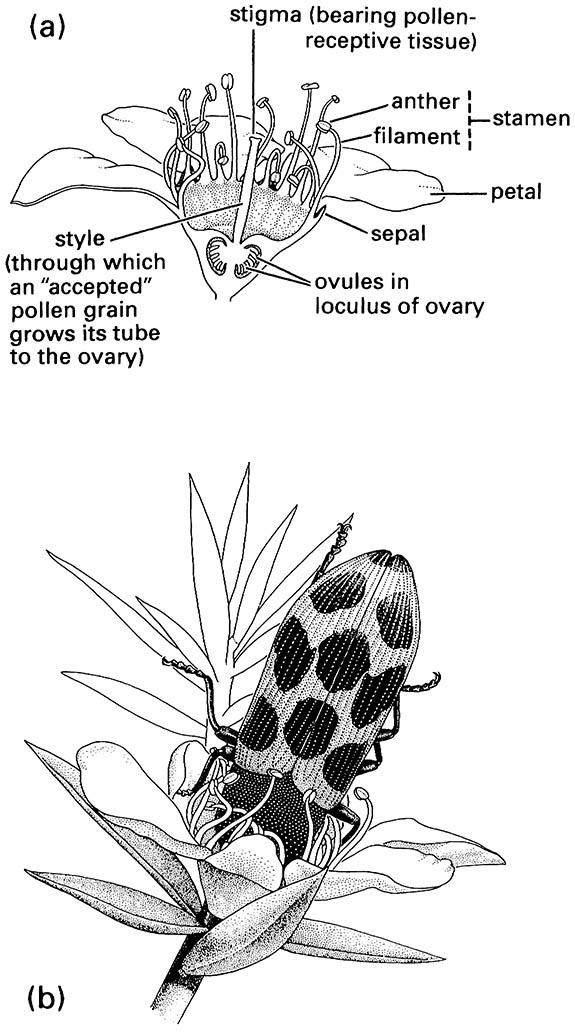
(a) diagram of a flower showing the parts; (b) a jewel beetle, Stigmodera sp. (Coleoptera: Buprestidae), feeding from a flower.
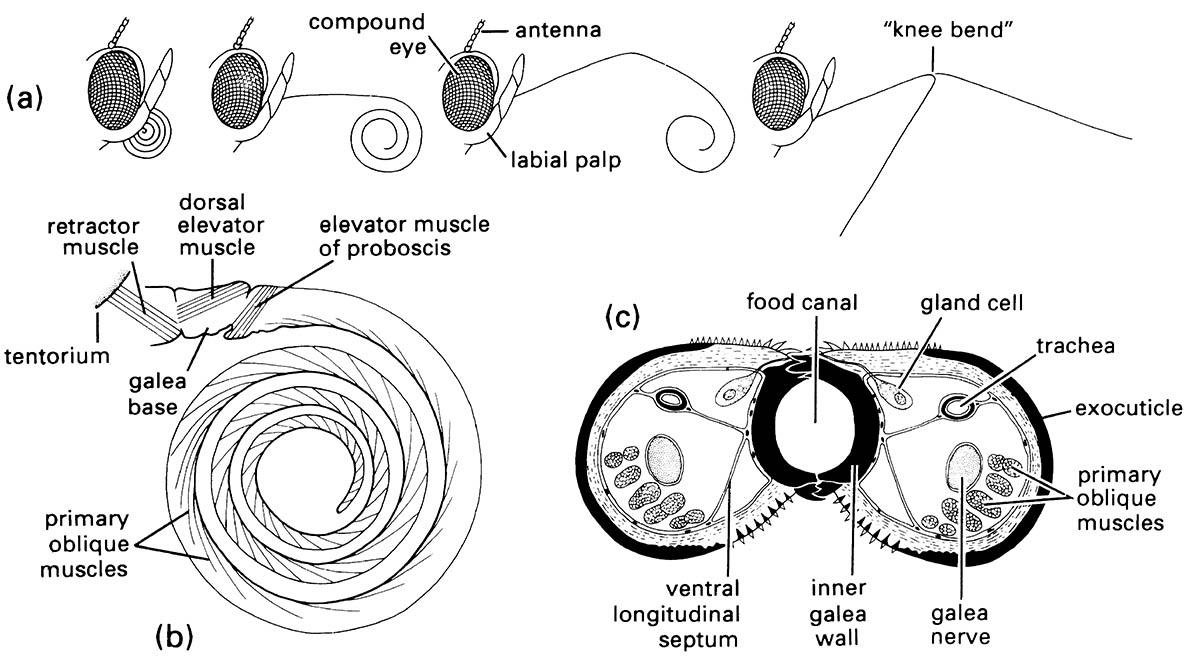
(a) Positions of the proboscis showing, from left to right, at rest, with proximal region uncoiling, with distal region uncoiling, and fully extended with tip in two of many possible different positions due to flexing at «knee bend». (b) Lateral view of proboscis musculature. (c) Transverse section of the proboscis in the proximal region. (After Eastham & Eassa 1955)
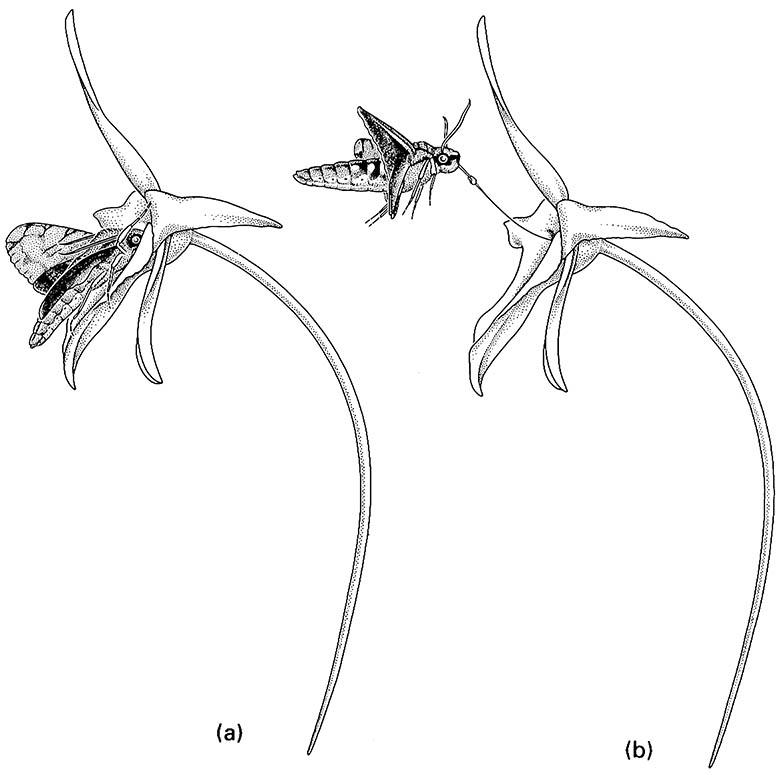
(a) full insertion of the moth’s proboscis; (b) upward flight during withdrawal of the proboscis with the orchid pollinium attached. (After Wasserthal 1997)

