13.3.1. Host use by parasitoids
Parasitoids require only a single individual in which to complete development, they always kill their immature host, and rarely are parasitic in the adult stage. Insect- eating (entomophagous) parasitoids show a range of strategies for development on their selected insect hosts. The larva may be ectoparasitic, developing externally, or endoparasitic, developing within the host. Eggs (or larvae) of ectoparasitoids are laid close to or upon the body of the host, as are sometimes those of endoparasitoids. However, in the latter group, more often the eggs are laid within the body of the host, using a piercing ovipositor (in hymenopterans) or a substitutional ovipositor (in parasitoid dipterans). Certain parasitoids that feed within host pupal cases or under the covers and protective cases of scale insects and the like actually are ectophages (external feeding), living internal to the protection but external to the insect host body. These different feeding modes give different exposures to the host immune system, with endoparasitoids encountering and ectoparasitoids avoiding the host defenses (section 13.2.3). Ectoparasitoids are often less host specific than endoparasitoids, as they have less intimate association with the host than do endoparasitoids, which must counter the species-specific variations of the host immune system.
Parasitoids may be solitary on or in their host, or gregarious. The number of parasitoids that can develop on a host relates to the size of the host, its postinfected longevity, and the size (and biomass) of the parasitoid. Development of several parasitoids in one individual host arises commonly through the female ovipositing several eggs on a single host, or, less often, by polyembryony, in which a single egg laid by the mother divides and can give rise to numerous offspring (section 5.10.3). Gregarious parasitoids appear able to regulate the clutch size in relation to the quality and size of the host.
Most parasitoids host discriminate; i.e. they can recognize, and generally reject, hosts that are parasitized already, either by themselves, their conspecifics, or another species. Distinguishing unparasitized from parasitized hosts generally involves a marking pheromone placed internally or externally on the host at the time of oviposition.
However, not all parasitoids avoid already parasitized hosts. In superparasitism, a host receives multiple eggs either from a single individual or from several individuals of the same parasitoid species; although the host cannot sustain the total parasitoid burden to maturity. The outcome of multiple oviposition is discussed in section 13.3.2. Theoretical models, some of which have been substantiated experimentally, imply that superparasitism will increase:
- as unparasitized hosts are depleted;
- as parasitoid numbers searching any patch increase;
- in species with high fecundity and small eggs.
Although historically all such instances were deemed to have been “mistakes”, there is some evidence of adaptive benefits deriving from the strategy. Super-parasitism is adaptive for individual parasitoids when there is competition for scarce hosts, but avoidance is adaptive when hosts are abundant. Very direct benefits accrue in the case of a solitary parasitoid that uses a host that is able to encapsulate a parasitoid egg (section 13.2.3). Here, a first-laid egg may use all the host hemocytes, and a subsequent egg may thereby escape encapsulation.
In multiparasitism, a host receives eggs of more than one species of parasitoid. Multiparasitism occurs more often than superparasitism, perhaps because parasitoid species are less able to recognize the marking pheromones placed by species other than their own. Closely related parasitoids may recognize each others’ marks, whereas more distantly related species may be unable to do so. However, secondary parasitoids, called hyperparasitoids, appear able to detect the odors left by a primary parasitoid, allowing accurate location of the site for the development of the hyperparasite.
Hyperparasitic development involves a secondary parasitoid developing at the expense of the primary parasitoid. Some insects are obligate hyperparasitoids, developing only within primary parasitoids, whereas others are facultative and may develop also as primary parasitoids. Development may be external or internal to the primary parasitoid host, with oviposition into the primary host in the former, or into the primary parasitoid in the latter (Fig. 13.6). External feeding is frequent, and hyperparasitoids are predominantly restricted to the host larval stage, sometimes the pupa; hyperparasitoids of eggs and adults of primary parasitoid hosts are very rare.
Hyperparasitoids belong to two families of Diptera (certain Bombyliidae and Conopidae), two families of Coleoptera (a few Rhipiphoridae and Cleridae), and notably the Hymenoptera, principally amongst 11 families of the superfamily Chalcidoidea, in four subfamilies of Ichneumonidae, and in Figitidae (Cynipoidea). Hyperparasitoids are absent among the Tachinidae and surprisingly do not seem to have evolved in certain parasitic wasp families such as Braconidae, Trichogrammatidae, and Mymaridae. Within the Hymenoptera, hyperparasitism has evolved several times, each originating in some manner from primary parasitism, with facultative hyperparasitism demonstrating the ease of the transition. Hymenopteran hyperparasitoids attack a wide range of hymenopteran-parasitized insects, predominantly amongst the hemipterans (especially Sternorrhyncha), Lepidoptera, and symphytans. Hyper-parasitoids often have a broader host range than the frequently oligophagous or monophagous primary parasitoids. However, as with primary parasitoids, endophagous hyperparasitoids seem to be more host specific than those that feed externally, relating to the greater physiological problems experienced when developing within another living organism. Additionally, foraging and assessment of host suitability of a complexity comparable with that of primary parasitoids is known, at least for cynipoid hyperparasitoids of aphidophagous parasitoids (Fig. 13.7). As explained in section 16.5.1, hyperparasitism and the degree of host-specificity is fundamental information in biological control programs.
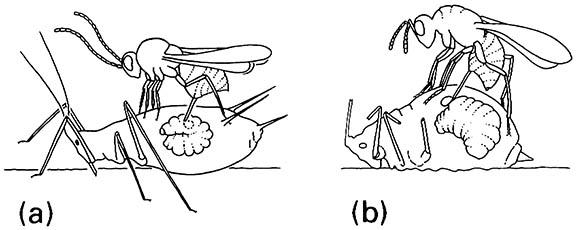
(a) endophagous Alloxysta victrix (Hymenoptera: Figitidae) ovipositing into a primary parasitoid inside a live aphid; (b) ectophagous Asaphes lucens (Hymenoptera: Pteromalidae) ovipositing onto a primary parasitoid in a mummified aphid. (After Sullivan 1988)
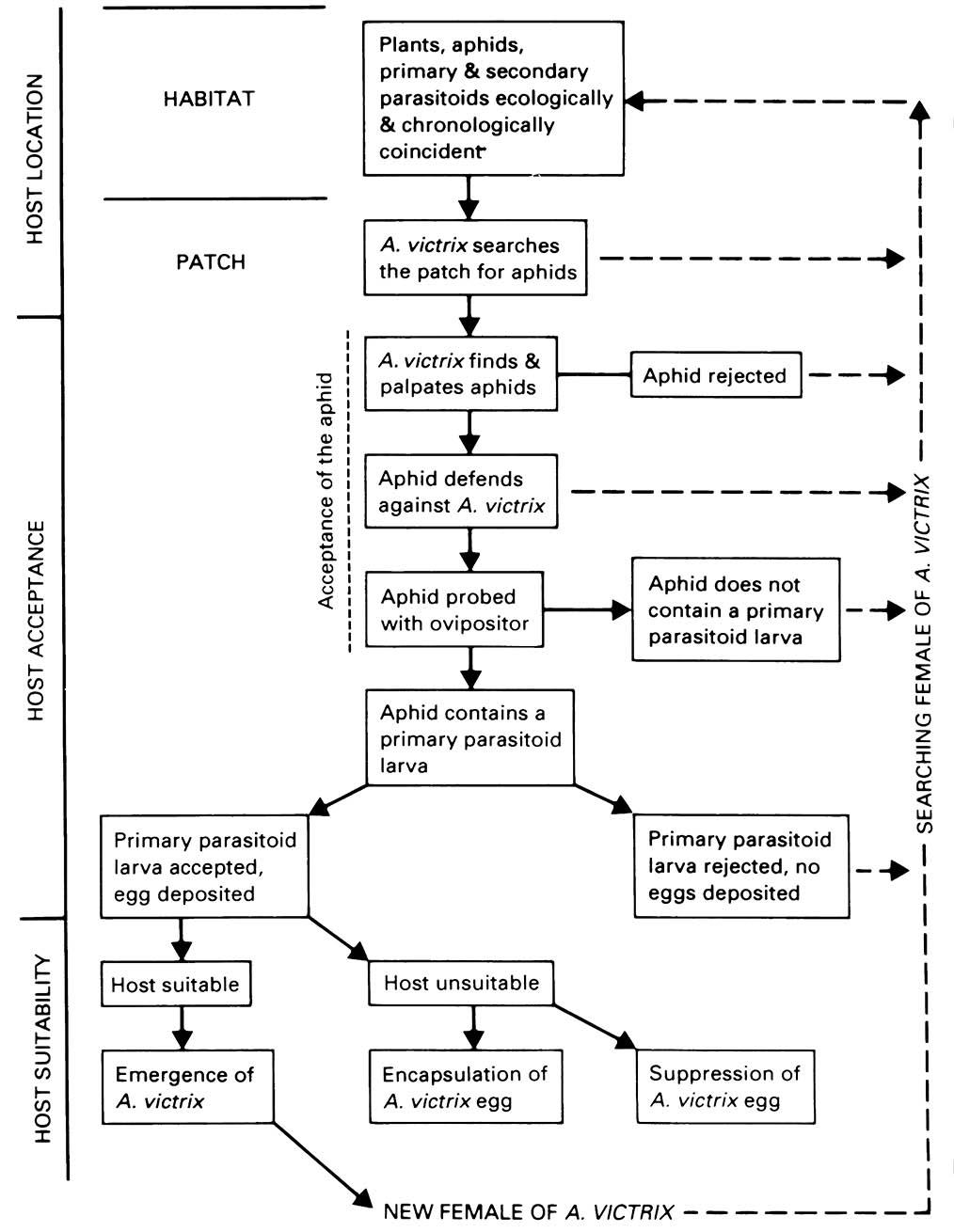
(After Gutierrez 1970)

