11.2.3. Sap sucking
The feeding activities of insects that chew or mine leaves and shoots cause obvious damage. In contrast, structural damage caused by sap-sucking insects often is inconspicuous, as the withdrawal of cell contents from plant tissues usually leaves the cell walls intact. Damage to the plant may be difficult to quantify even though the sap sucker drains plant resources (by removing phloem or xylem contents), causing loss of condition such as retarded root growth, fewer leaves, or less overall biomass accumulation compared with unaffected plants. These effects may be detectable with confidence only by controlled experiments in which the growth of infested and uninfected plants is compared. Certain sap-sucking insects do cause conspicuous tissue necrosis either by transmitting diseases, especially viral ones, or by injecting toxic saliva, whereas others induce obvious tissue distortion or growth abnormalities called galls (section 11.2.4).
Most sap-sucking insects belong to the Hemiptera. All hemipterans have long, thread-like mouthparts consisting of appressed mandibular and maxillary stylets forming a bundle lying in a groove in the labium (Box 11.8). The maxillary stylet contains a salivary canal that directs saliva into the plant, and a food canal through which plant juice or sap is sucked up into the insect’s gut. Only the stylets enter the tissues of the host plant (Fig. 11.4a). They may penetrate superficially into a leaf or deeply into a plant stem or leaf midrib, following either an intracellular or inter- cellular path, depending on species. The feeding site reached by the stylet tips may be in the parenchyma (e.g. some immature scale insects, many Heteroptera), the phloem (e.g. most aphids, mealybugs, soft scales, psyllids, and leafhoppers), or the xylem (e.g. spittle bugs and cicadas). In addition to a hydrolyzing type of saliva, many species produce a solidifying saliva that forms a sheath around the stylets as they enter and penetrate the plant tissue. This sheath can be stained in tissue sections and allows the feeding tracks to be traced to the feeding site (Fig. 11.4b,c). The two feeding strategies of hemipterans, stylet-sheath and macerate-and-flush feeding, are described in section 3.6.2, and the gut specializations of hemipterans for dealing with a watery diet are discussed in Box 3.3. Many species of plant-feeding Hemiptera are considered serious agricultural and horticultural pests. Loss of sap leads to wilting, distortion, or stunting of shoots. Movement of the insect between host plants can lead to the efficient transmission of plant viruses and other diseases, especially by aphids and whiteflies. The sugary excreta (honeydew) of phloem-feeding Hemiptera, particularly coccoids, is used by black sooty molds, which soil leaves and fruits and can impair photosynthesis.
Thrips (Thysanoptera) that feed by sucking plant juices penetrate the tissues using their stylets (Fig. 2.13) to pierce the epidermis and then rupture individual cells below. Damaged areas discolor and the leaf, bud, flower, or shoot may wither and die. Plant damage typically is concentrated on rapidly growing tissues, so that flowering and leaf flushing may be seriously disrupted. Some thrips inject toxic saliva during feeding or transmit viruses, such as the Tospovirus (Bunyaviridae) carried by the pestiferous western flower thrips, Frankliniella occidentalis. A few hundred thrips species have been recorded attacking cultivated plants, but only 10 species transmit tospoviruses.
Outside the Hemiptera and Thysanoptera, the sap-sucking habit is rare in extant insects. Many fossil species, however, had a rostrum with piercing-and-sucking mouthparts. Palaeodictyopteroids (Fig. 8.2), for example, probably fed by imbibing juices from plant organs.
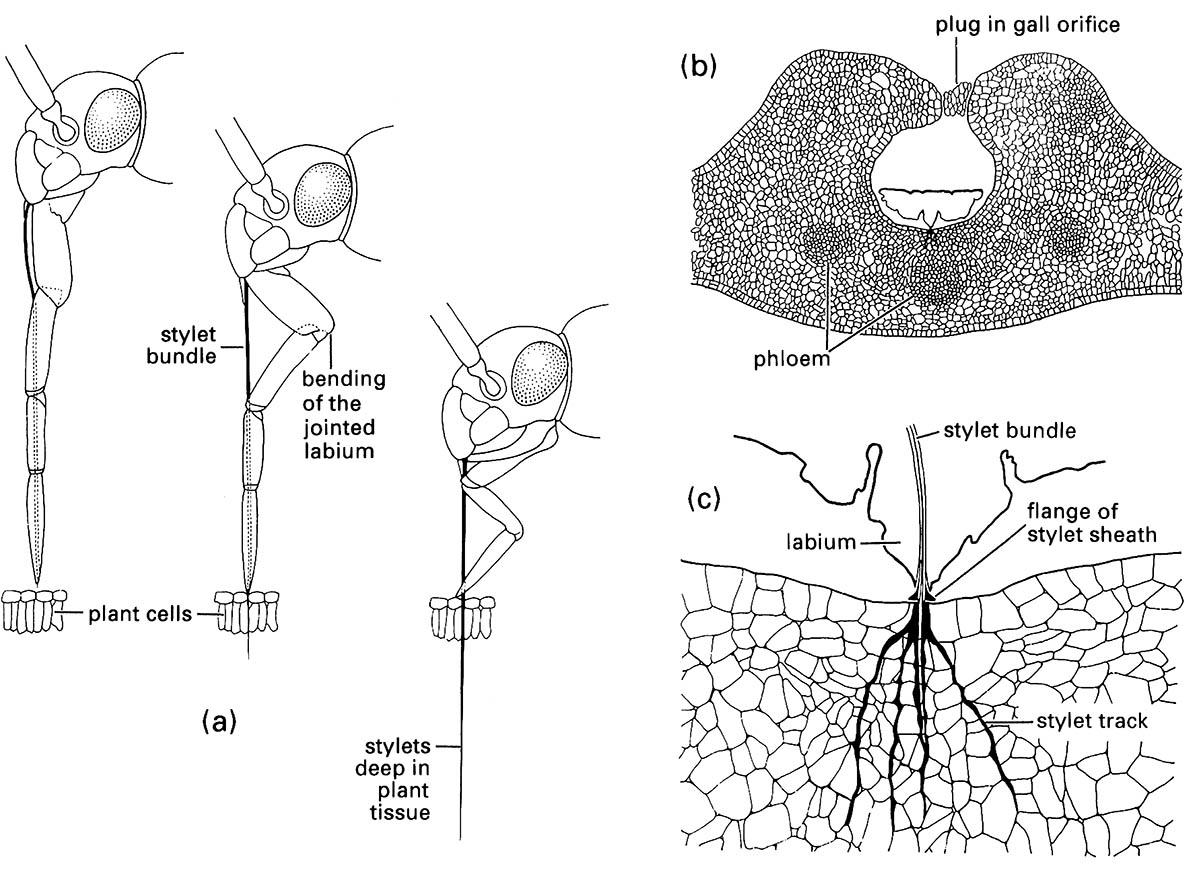
(a) penetration of plant tissue by a mirid bug showing bending of the labium as the stylets enter the plant; (b) transverse section through a eucalypt leaf gall containing a feeding nymph of a scale insect, Apiomorpha (Eriococcidae); (c) enlargement of the feeding site of (b) showing multiple stylet tracks (formed of solidifying saliva) resulting from probing of the parenchyma. ((a) After Poisson 1951)
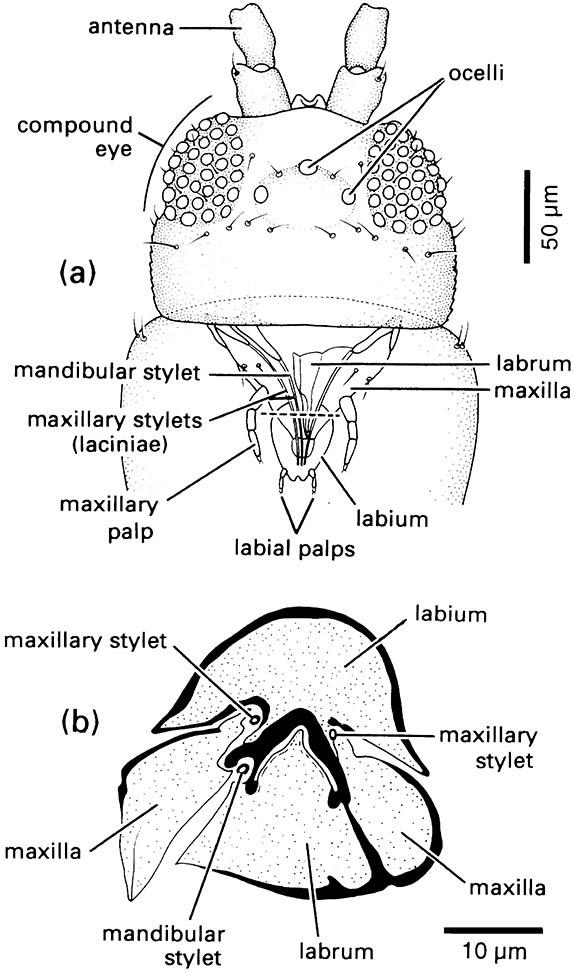
(a) Dorsal view of head showing mouthparts through prothorax. (b) Transverse section through proboscis. The plane of the transverse section is indicated by the dashed line in (a). (After Matsuda 1965; CSIRO 1970)
(After Kukalová 1970)

