5.8. Oviparity (egg-laying)
The vast majority of female insects are oviparous, i.e. they lay eggs. Generally, ovulation — expulsion of eggs from the ovary into the oviducts — is followed rapidly by fertilization and then oviposition. Ovulation is con- trolled by hormones released from the brain, whereas oviposition appears to be under both hormonal and neural control. Oviposition, the process of the egg passing from the external genital opening or vulva to the outside of the female (Fig. 5.9), is often associated with behaviors such as digging or probing into an egg- laying site, but often the eggs are simply dropped to the ground or into water. Usually the eggs are deposited on or near the food required by the offspring upon hatching. Care of eggs after laying often is lacking or minimal, but social insects (Chapter 12) have highly developed care, and certain aquatic insects show very unusual paternal care (Box 5.5).
An insect egg within the female’s ovary is complete when an oocyte becomes covered with an outer protective coating, the eggshell, formed of the vitelline membrane and the chorion. The chorion may be composed of any or all of the following layers: wax layer, innermost chorion, endochorion, and exochorion (Fig. 5.10). Ovarian follicle cells produce the eggshell and the surface sculpturing of the chorion usually reflects the outline of these cells. Typically, the eggs are yolk-rich and thus large relative to the size of the adult insect; egg cells range in length from 0.2 mm to about 13 mm. Embryonic development within the egg begins after egg activation (section 6.2.1).
The eggshell has a number of important functions. Its design allows selective entry of the sperm at the time of fertilization (section 5.6). Its elasticity facilitates oviposition, especially for species in which the eggs are compressed during passage down a narrow egg-laying tube, as described below. Its structure and composition afford the embryo protection from deleterious conditions such as unfavorable humidity and temperature, and microbial infection, while also allowing the exchange of oxygen and carbon dioxide between the inside and outside of the egg.
The differences seen in composition and complexity of layering of the eggshell in different insect groups generally are correlated with the environmental conditions encountered at the site of oviposition. In parasitic wasps the eggshell is usually thin and relatively homogeneous, allowing flexibility during passage down the narrow ovipositor, but, because the embryo develops within host tissues where desiccation is not a hazard, the wax layer of the eggshell is absent. In contrast, many insects lay their eggs in dry places and here the problem of avoiding water loss while obtaining oxygen is often acute because of the high surface area to volume ratio of most eggs. The majority of terrestrial eggs have a hydrofuge waxy chorion that contains a meshwork holding a layer of gas in contact with the outside atmosphere via narrow holes, or aeropyles.
The females of many insects (e.g. Zygentoma, many Odonata, Orthoptera, some Hemiptera, some Thysano- ptera, and Hymenoptera) have appendages of the eighth and ninth abdominal segments modified to form an egg-laying organ or ovipositor (section 2.5.1). In other insects (e.g. many Lepidoptera, Coleoptera, and Diptera) it is the posterior segments rather than appendages of the female’s abdomen that function as an ovipositor (a “substitutional” ovipositor). Often these segments can be protracted into a telescopic tube in which the opening of the egg passage is close to the distal end. The ovipositor or the modified end of the abdomen enables the insect to insert its eggs into particular sites, such as into crevices, soil, plant tissues, or, in the case of many parasitic species, into an arthropod host. Other insects, such as Isoptera, Phthiraptera, and many Plecoptera, lack an egg-laying organ and eggs are deposited simply on a surface.
In certain Hymenoptera (some wasps, bees, and ants) the ovipositor has lost its egg-laying function and is used as a poison-injecting sting. The stinging Hymenoptera eject the eggs from the opening of the genital chamber at the base of the modified ovipositor. However, in most wasps the eggs pass down the canal of the ovipositor shaft, even if the shaft is very narrow (Fig. 5.11). In some parasitic wasps with very slender ovipositors the eggs are extremely compressed and stretched as they move through the narrow canal of the shaft.
The valves of an insect ovipositor usually are held together by interlocking tongue-and-groove joints, which prevent lateral movement but allow the valves to slide back and forth on one another. Such movement, and sometimes also the presence of serrations on the tip of the ovipositor, is responsible for the piercing action of the ovipositor into an egg-laying site. Movement of eggs down the ovipositor tube is possible because of many posteriorly directed “scales” (micro-sculpturing) located on the inside surface of the valves. Ovipositor scales vary in shape (from plate-like to spine-like) and in arrangement among insect groups, and are seen best under the scanning electron microscope.
The scales found in the conspicuous ovipositors of crickets and katydids exemplify these variations (Orthoptera: Gryllidae and Tettigoniidae). The ovipositor of the field cricket Teleogryllus commodus (Fig. 5.12) possesses overlapping plate-like scales and scattered, short sensilla along the length of the egg canal. These sensilla may provide information on the position of the egg as it moves down the canal, whereas a group of larger sensilla at the apex of each dorsal valve presumably signals that the egg has been expelled. In addition, in T. commodus and some other insects, there are scales on the outer surface of the ovipositor tip, which are orientated in the opposite direction to those on the inner surface. These are thought to assist with penetration of the substrate and holding the ovipositor in position during egg-laying.
In addition to the eggshell, many eggs are provided with a proteinaceous secretion or cement which coats and fastens them to a substrate, such as a vertebrate hair in the case of sucking lice, or a plant surface in the case of many beetles (Fig. 5.9). Colleterial glands, accessory glands of the female reproductive tract, produce such secretions. In other insects, groups of thin-shelled eggs are enclosed in an ootheca, which protects the developing embryos from desiccation. The colleterial glands produce the tanned, purse-like ootheca of cockroaches (Box 9.8) and the frothy ootheca of mantids (see Plate 3.3, here), whereas the foamy ootheca that surrounds locust and other orthopteran eggs in the soil is formed from the accessory glands in conjunction with other parts of the reproductive tract.
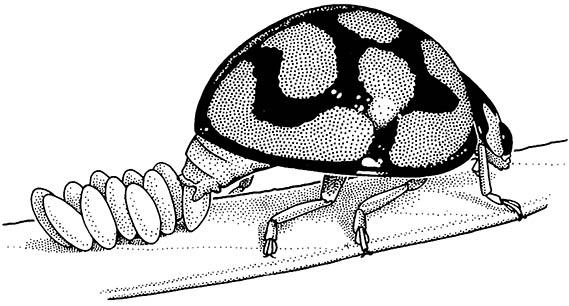
The eggs adhere to the leaf surface because of a sticky secretion applied to each egg. (After Blaney 1976)
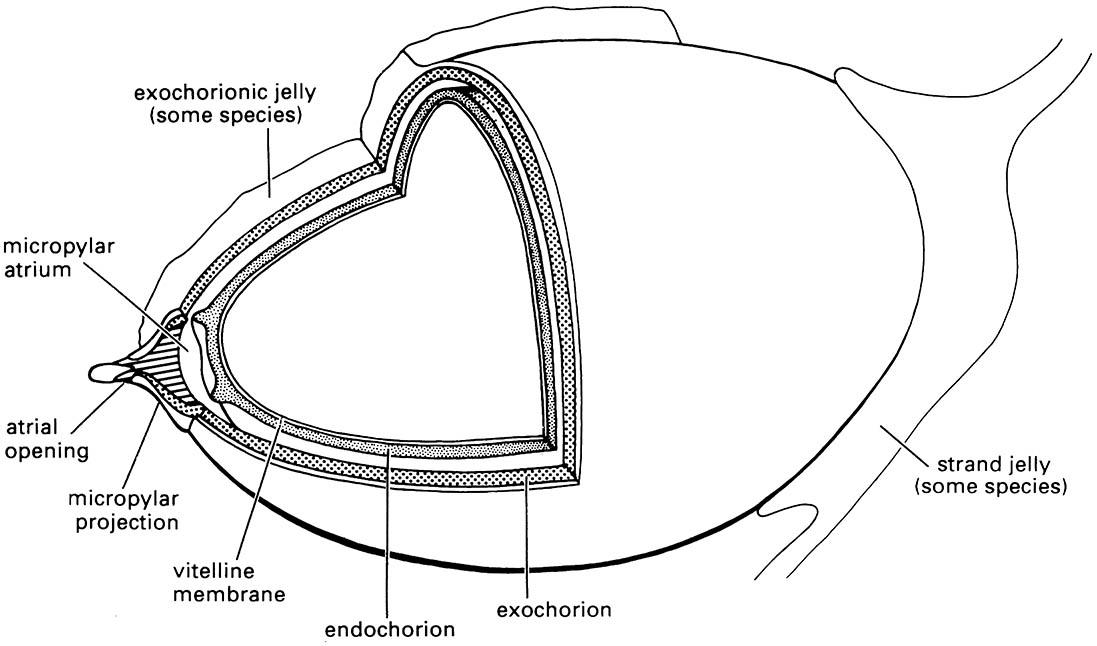
Libelluloid dragonflies oviposit into freshwater but always exophytically (i.e. outside of plant tissues). The endochorionic and exochorionic layers of the eggshell are separated by a distinct gap in some species. A gelatinous matrix may be present on the exochorion or as connecting strands between eggs. (After Trueman 1991)
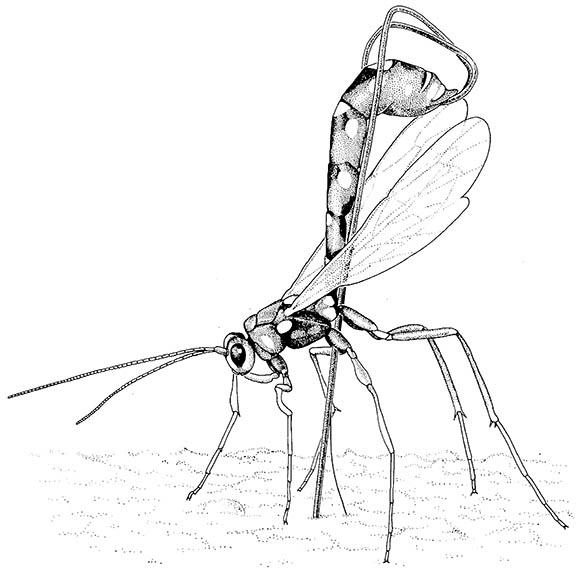
If a larva is located, she stings and paralyses it before laying an egg on it.
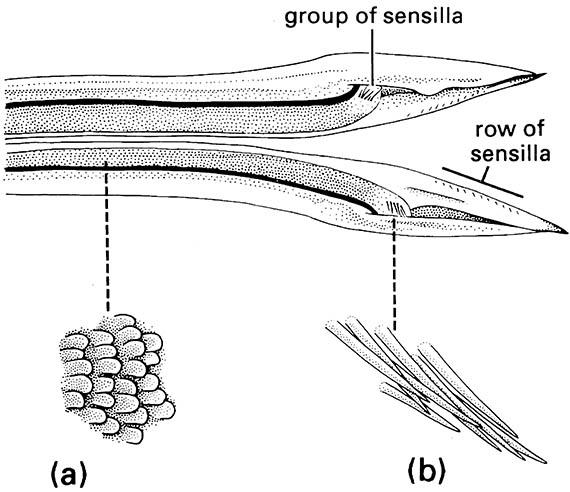
Enlargements show: (a) posteriorly directed ovipositor scales; (b) distal group of sensilla. (After Austin & Browning 1981)

