5.3. Sexual selection
Many insects are sexually dimorphic, usually with the male adorned with secondary sexual characteristics, some of which have been noted above in relation to courtship display. In many insect mating systems courtship can be viewed as intraspecific competition for mates, with certain male behaviors inducing female response in ways that can increase the mating success of particular males. Because females differ in their responsiveness to male stimuli, females can be said to choose between mates, and courtship thus is competitive. Female choice might involve no more than selection of the winners of male—male interactions, or may be as subtle as discrimination between the sperm of different males (section 5.7). All elements of communication associated with gaining fertilization of the female, from long-distance sexual calling through to insemination, are seen as competitive courtship between males. By this reasoning, members of a species avoid hybrid matings because of a specific-mate recognition system that evolved under the direction of female choice, rather than as a mechanism to promote species cohesion.
Understanding sexual dimorphism in insects such as staghorn beetles, song in orthopterans and cicadas, and wing color in butterflies and odonates helped Darwin to recognize the operation of sexual selection — the elaboration of features associated with sexual competition rather than directly with survival. Since Darwin’s day, studies of sexual selection often have featured insects because of their short generation time, facility of manipulation in the laboratory, and relative ease of observation in the field. For example, dung beetles belonging to the large and diverse genus Onthophagus may display elaborate horns that vary in size between individuals and in position on the body between species. Large horns are restricted nearly exclusively to males, with only one species known in which the female has better developed protuberances than conspecific males. Studies show that females preferentially select males with larger horns as mates. Males size each other up and may fight, but there is no lek. Benefits to the female come from long-horned males’ better defensive capabilities against intruders seeking to oust the resident from the resource-rich nest site, provisioned with dung, his mate, and their young (Fig. 9.5). However, the system is more complicated, at least in the North American Onthophagus taurus. In this dung beetle, male horn size is dimorphic, with insects greater than a certain threshold size having large horns, and those below a certain size having only minimal horns (Fig. 5.2). However, nimble small-horned males attain some mating success through sneakily circumventing the large-horned but clumsy male defending the tunnel entrance, either by evasion or by digging a side tunnel to access the female.
Darwin could not understand why the size and location of horns varied, but now elegant comparative studies have shown that elaboration of large horns bears a developmental cost. Organs located close to a large horn are diminished in size — evidently resources are reallocated during development so that either eyes, antennae, or wings apparently “pay for” being close to a male’s large horn. Regular-sized adjacent organs are developed in females of the same species with smaller horns and male conspecifics with weakly developed horns. Exceptionally, the species with the female having long horns on the head and thorax commensurately has reduced adjacent organs, and a sex reversal in defensive roles is assumed to have taken place. The different locations of the horns appear to be explained by selective sacrifice of adjacent organs according to species behavior. Thus, nocturnal species that require good eyes have their horns located elsewhere than the head; those requiring flight to locate dispersed dung have horns on the head where they interfere with eye or antennal size, but do not compromise the wings. Presumably, the upper limit to horn elaboration either is the burden of ever-increasing deleterious effects on adjacent vital functions, or an upper limit on the volume of new cuticle that can develop sub-epidermally in the pharate pupa within the final-instar larva, under juvenile hormonal control.
Size alone may be important in female choice: in some stick-insects (also called walking sticks) larger males often monopolize females. Males fight over their females by boxing at each other with their legs while grasping the female’s abdomen with their claspers (as shown for Diapheromera veliei in the vignette for this chapter). Ornaments used in male-to-male combat include the extraordinary “antlers” of Phytalmia (Tephritidae) (Fig. 5.3) and the eyestalks of a few other flies (such as Diopsidae), which are used in competition for access to the oviposition site visited by females. Furthermore, in studied species of diopsid (stalk-eyed flies), female mate choice is based on eyestalk length up to a dimension of eye separation that can surpass the body length. Cases such as these provide evidence for two apparently alternative but likely non-exclusive explanations for male adornments: sexy sons or good genes. If the female choice commences arbitrarily for any particular adornment, their selection alone will drive the increased frequency and development of the elaboration in male offspring in ensuing generations (the sexy sons) despite countervailing selection against conventional unfitness. Alternatively, females may choose mates that can demonstrate their fitness by carrying around apparently deleterious elaborations thereby indicating a superior genetic background (good genes). Darwin’s interpretation of the enigma of female choice certainly is substantiated, not least by studies of insects.
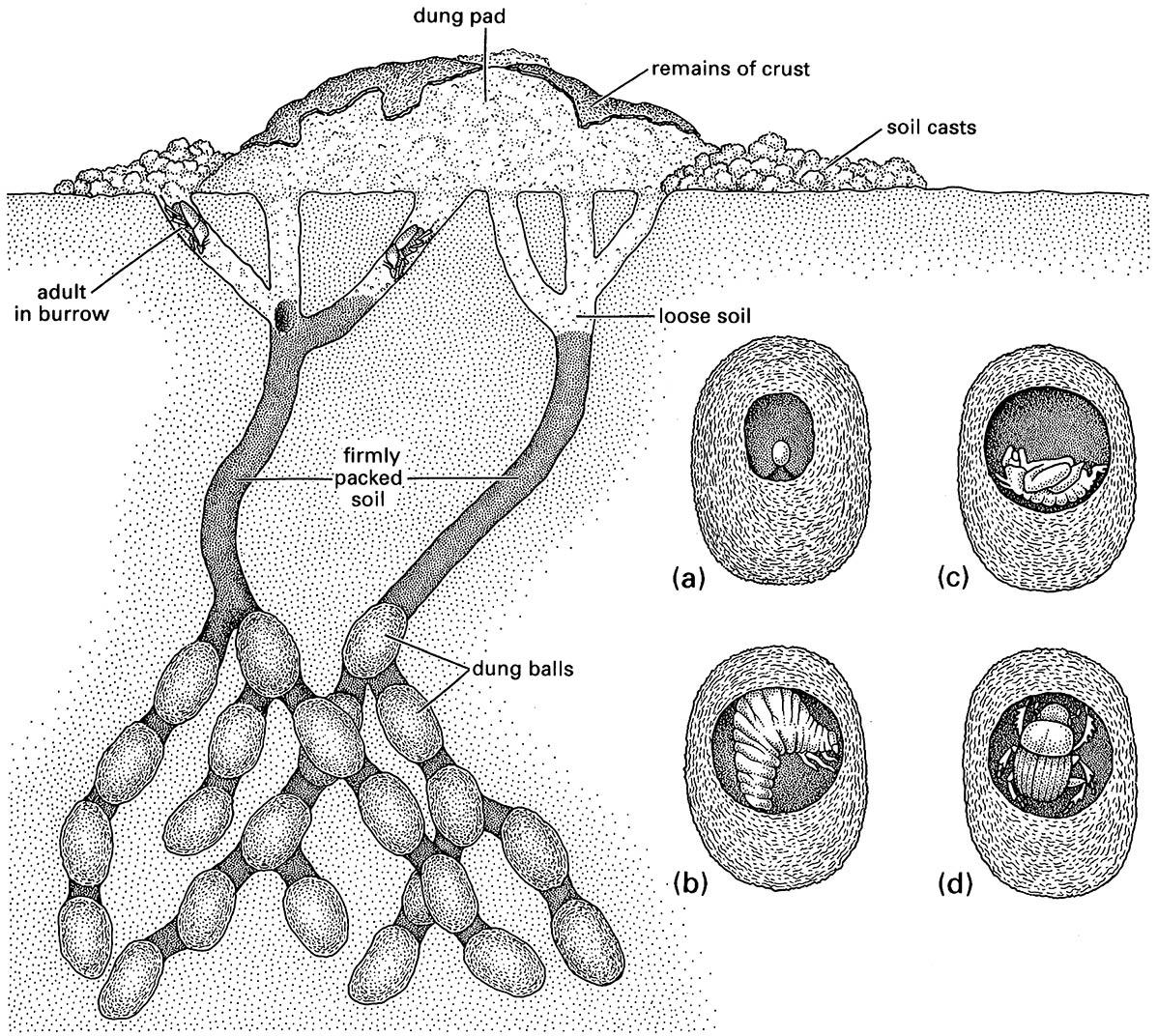
The inset shows an individual dung ball within which beetle development takes place: (a) egg; (b) larva, which feeds on the dung; (c) pupa; and (d) adult just prior to emergence. (After Waterhouse 1974)
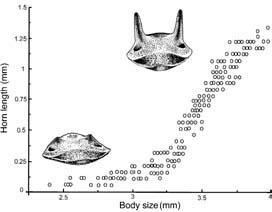
(After Moczek & Emlen 2000; with beetle heads drawn by S.L. Thrasher)
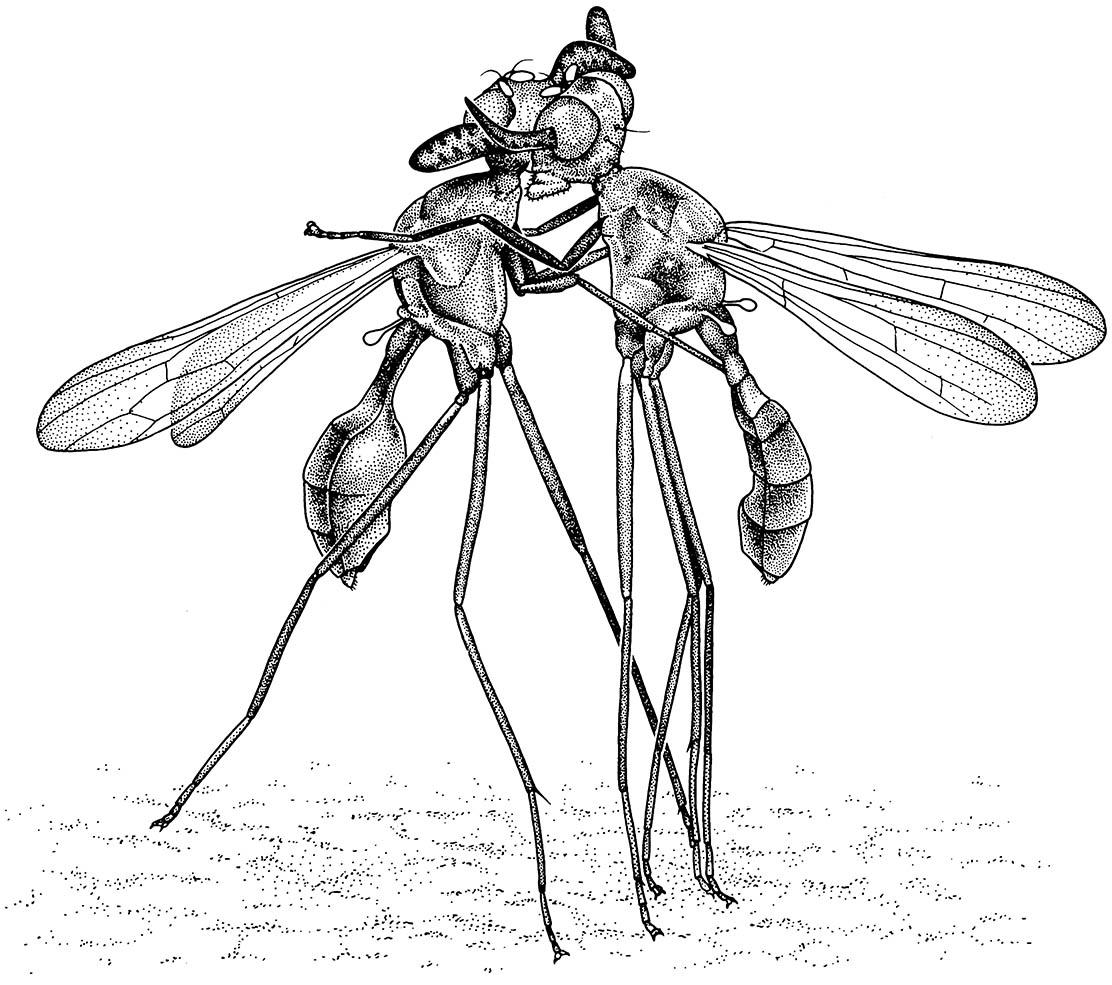
These tropical rainforest flies thus have a resource-defense mating system. (After Dodson 1989, 1997)

