5.5. Diversity in genitalic morphology
The components of the terminalia of insects are very diverse in structure and frequently exhibit species-specific morphology (Fig. 5.5), even in otherwise similar species. Variations in external features of the male genitalia often allow differentiation of species, whereas external structures in the female usually are simpler and less varied. Conversely, the internal genitalia of female insects often show greater diagnostic variability than the internal structures of the males. However, recent development of techniques to evert the endophallus of the male aedeagus allows increasing demonstration of the species-specific shapes of these male internal structures. In general, external genitalia of both sexes are much more sclerotized than the internal genitalia, although parts of the reproductive tract are lined with cuticle. Increasingly, characteristics of insect internal genitalia and even soft tissues are recognized as allowing species delineation and providing evidence of phylogenetic relationships.
Observations that genitalia frequently are complex and species-specific in form, sometimes appearing to correspond tightly between the sexes, led to formulation of the “lock-and-key” hypothesis as an explanation of this phenomenon. Species-specific male genitalia (the “keys”) were believed to fit only the conspecific female genitalia (the “locks”), thus preventing interspecific mating or fertilization. For example, in some katydids interspecific copulations are unsuccessful in transmitting spermatophores because the specific structure of the male claspers (modified cerci) fails to fit the subgenital plate of the “wrong” female. The lock-and-key hypothesis was postulated first in 1844 and has been the subject of controversy ever since. In many (but not all) insects, mechanical exclusion of “incorrect” male genitalia by the female is seen as unlikely for several reasons:
- morphological correlation between conspecific male and female parts may be poor;
- interspecific, intergeneric, and even interfamilial hybrids can be induced;
- amputation experiments have demonstrated that male insects do not need all parts of the genitalia to inseminate conspecific females successfully.
Some support for the lock-and-key hypothesis comes from studies of certain noctuid moths in which structural correspondence in the internal genitalia of the male and female is thought to indicate their function as a postcopulatory but prezygotic isolating mechanism. Laboratory experiments involving interspecific matings support a lock-and-key function for the internal structures of other noctuid moths. Interspecific copulation can occur, although without a precise fit of the male’s vesica (the flexible tube everted from the aedeagus during insemination) into the female’s bursa (genital pouch); the sperm may be discharged from the spermatophore to the cavity of the bursa, instead of into the duct that leads to the spermatheca, resulting in fertilization failure. In conspecific pairings, the spermatophore is positioned so that its opening lies opposite that of the duct (Fig. 5.6).
In species of Japanese ground beetle of the genus Carabus (subgenus Ohomopterus) (Carabidae), the male’s copulatory piece (a part of the endophallus) is a precise fit for the vaginal appendix of the conspecific female. During copulation, the male everts his endophallus in the female’s vagina and the copulatory piece is inserted into the vaginal appendix. Closely related parapatric species are of similar size and external appearance but their copulatory piece and vaginal appendix are very different in shape. Although hybrids occur in areas of overlap of species, matings between different species of beetles have been observed to result in broken copulatory pieces and ruptured vaginal membranes, as well as reduced fertilization rates compared with conspecific pairings. Thus, the genital lock-and-key appears to select strongly against hybrid matings.
Mechanical reproductive isolation is not the only available explanation of species-specific genital morphology. Five other hypotheses have been advanced: pleiotropy, genitalic recognition, female choice, inter- sexual conflict, and male—male competition. The first two of these are further attempts to account for reproductive isolation of different species, whereas the last three are concerned with sexual selection, a topic that is addressed in more detail in sections 5.3 and 5.7.
The pleiotropy hypothesis explains genitalic differences between species as chance effects of genes that primarily code for other vital characteristics of the organism. This idea fails to explain why genitalia should be more affected than other parts of the body. Nor can pleiotropy explain genital morphology in groups (such as the Odonata) in which organs other than the primary male genitalia have an intromittent function (like those on the anterior abdomen in odonates). Such secondary genitalia consistently become subject to the postulated pleiotropic effects whereas the primary genitalia do not, a result inexplicable by the pleiotropy hypothesis.
The hypothesis of genitalic recognition involves reproductive isolation of species via female sensory discrimination between different males based upon genitalic structures, both internal and external. The female thus responds only to the appropriate genital stimulation of a conspecific male and never to that of any male of another species.
In contrast, the female-choice hypothesis involves female sexual discrimination amongst conspecific males based on qualities that can vary intraspecifically and for which the female shows preference. This idea has nothing to do with the origin of reproductive isolation, although female choice may lead to reproductive isolation or speciation as a by-product. The female- choice hypothesis predicts diverse genitalic morphology in taxa with promiscuous females and uniform genitalia in strictly monogamous taxa. This prediction seems to be fulfilled in some insects. For example, in neotropical butterflies of the genus Heliconius, species in which females mate more than once are more likely to have species-specific male genitalia than species in which females mate only once. The greatest reduction in external genitalia (to near absence) occurs in termites, which, as might be predicted, form monogamous pairs.
Variation in genitalic and other body morphology also may result from intersexual conflict over control of fertilization. According to this hypothesis, females evolve barriers to successful fertilization in order to control mate choice, whereas males evolve mechanisms to overcome these barriers. For example, in many species of water-striders (Gerridae) males possess complex genital processes and modified appendages (Fig. 5.7) for grasping females, which in turn exhibit behaviors or morphological traits (e.g. abdominal spines) for dislodging males.
Another example is the long spermathecal tube of some female crickets (Gryllinae), fleas (Ceratophyllinae), flies (e.g. Tephritidae), and beetles (e.g. Chrysomelidae), which corresponds to a long spermatophore tube in the male, suggesting an evolutionary contest over control of sperm placement in the spermatheca. In the seed beetle Callosobruchus maculatus (Chrysomelidae: Bruchinae) spines on the male’s intromittent organ wound the genital tract of the female during copulation either to reduce remating and/or increase female oviposition rate, both of which would increase his fertilization success. The female responds by kicking to dislodge the male, thus shortening copulation time, reducing genital damage and presumably maintaining some control over fertilization. It is also possible that traumatic insemination (known in Cimicidae, including bed bugs Cimex lectularius), in which the male inseminates the female by piercing her body wall with his aedeagus, evolved as a mechanism for the male to short-circuit the normal insemination pathway controlled by the female. Such examples of apparent intersexual conflict could be viewed as male attempts to circumvent female choice.
Another possibility is that species-specific elaborations of male genitalia may result from interactions between conspecific males vying for inseminations. Selection may act on male genitalic clasping structures to prevent usurpation of the female during copulation or act on the intromittent organ itself to produce structures that can remove or displace the sperm of other males (section 5.7). However, although sperm displacement has been documented in a few insects, this phenomenon is unlikely to be a general explanation of male genitalic diversity because the penis of male insects often cannot reach the sperm storage organ(s) of the female or, if the spermathecal ducts are long and narrow, sperm flushing should be impeded.
Functional generalizations about the species-specific morphology of insect genitalia are controversial because different explanations no doubt apply in different groups. For example, male—male competition (via sperm removal and displacement; see Box 5.3) may be important in accounting for the shape of odonate penes, but appears irrelevant as an explanation in noctuid moths. Female choice, intersexual conflict, and male—male competition may have little selective effect on genitalic structures of insect species in which the female mates with only one male (as in termites). In such species, sexual selection may affect features that determine which male is chosen as a partner, but not how the male’s genitalia are shaped. Furthermore, both mechanical and sensory locks-and-keys will be unnecessary if isolating mechanisms, such as court- ship behavior or seasonal or ecological differences, are well developed. So we might predict morphological constancy (or a high level of similarity, allowing for some pleiotropy) in genitalic structures among species in a genus that has species-specific precopulatory displays involving non-genital structures followed by a single insemination of each female.
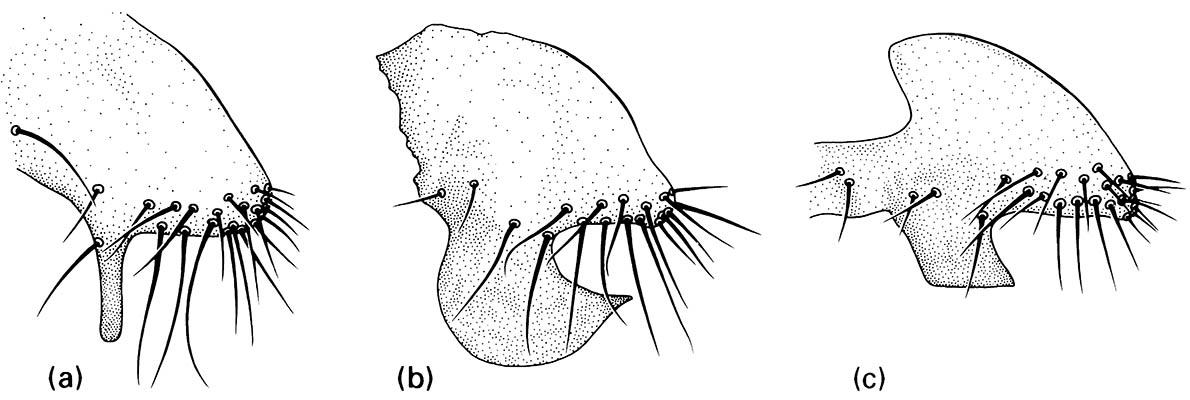
The epandrial processes of tergite 9 in: (a) D. mauritiana; (b) D. simulans; (c) D. melanogaster. (After Coyne 1983)
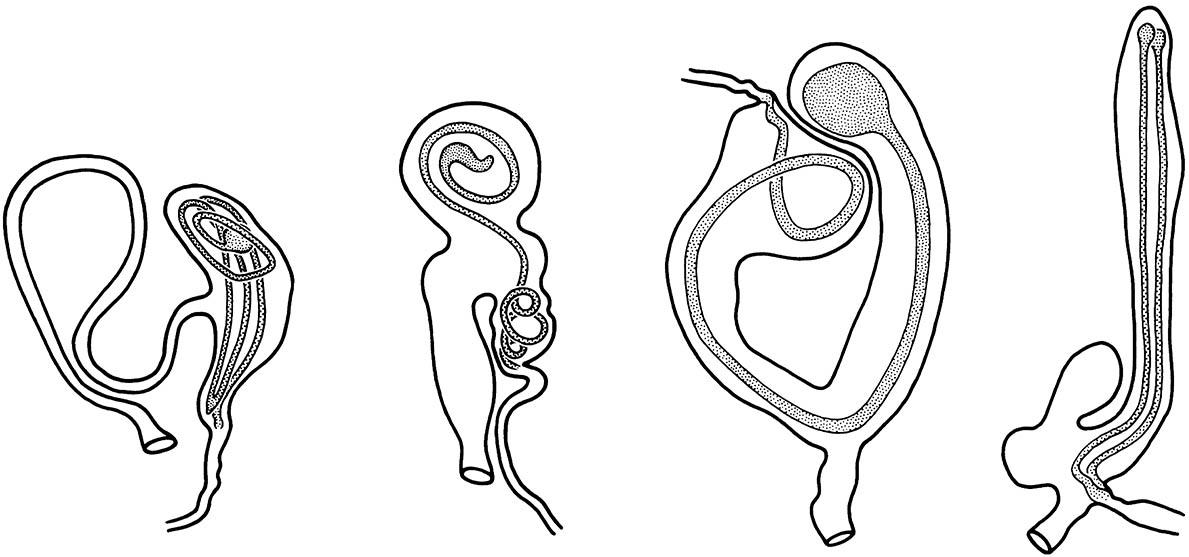
The sperm leave via the narrow end of each spermatophore, which has been deposited so that its opening lies opposite the “seminal duct” leading to the spermatheca (not drawn). The bursa on the far right contains two spermatophores, indicating that the female has remated. (After Williams 1941; Eberhard 1985)
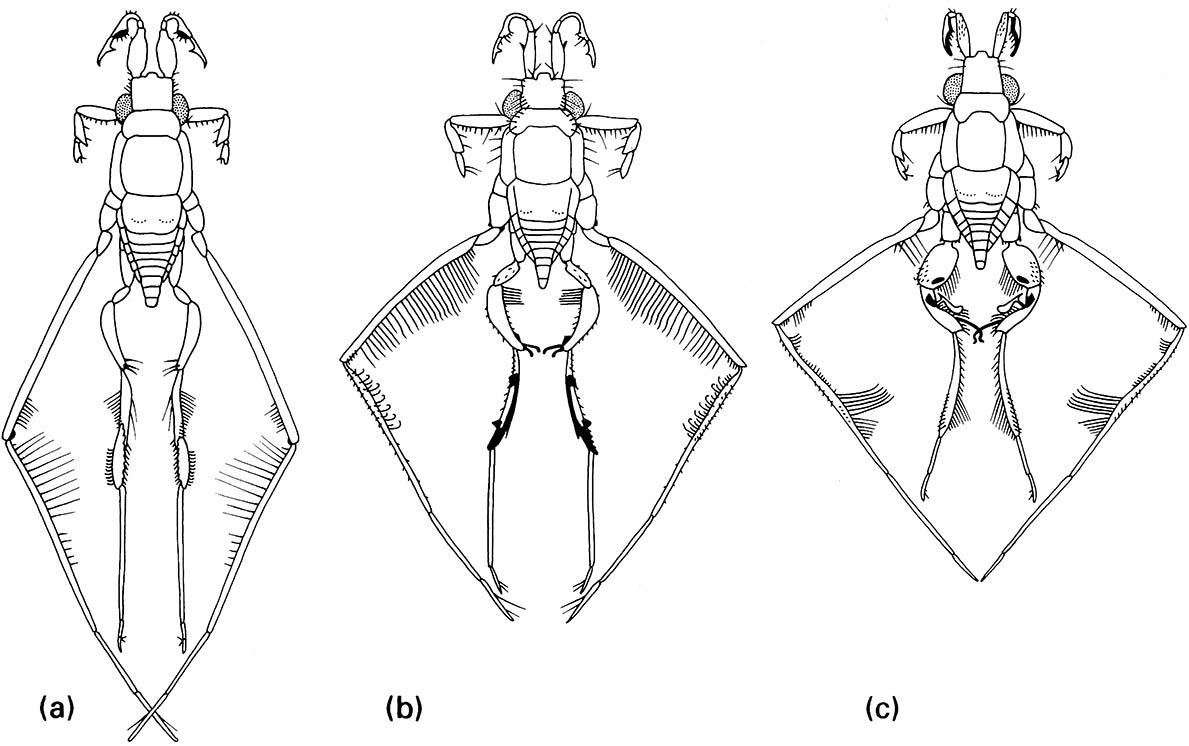
These non-genitalic male structures are specialized for contact with the female during mating, when the male rides on her back. Females of all species have a similar body form. (a) R. trulliger; (b) R. rileyi; (c) R. bergrothi. (After Hungerford 1954)

