5.11.1. Vitellogenesis and its regulation
In the ovary, both nurse cells (or trophocytes) and ovarian follicle cells are associated with the oocytes (section 3.8.1). These cells pass nutrients to the growing oocytes. The relatively slow period of oocyte growth is followed by a period of rapid yolk deposition, or vitellogenesis, which mostly occurs in the terminal oocyte of each ovariole and leads to the production of fully developed eggs. Vitellogenesis involves the production (mostly by the fat body) of specific female lipoglycoproteins called vitellogenins, followed by their passage into the oocyte. Once inside the oocyte these proteins are called vitellins and their chemical structure may differ slightly from that of vitellogenins. Lipid bodies — mostly triglycerides from the follicle cells, nurse cells, or fat body — also are deposited in the growing oocyte.
Vitellogenesis has been a favored area of insect hormone research because it is amenable to experimental manipulation with artificially supplied hormones, and analysis is facilitated by the large amounts of vitellogenins produced during egg growth. The regulation of vitellogenesis varies among insect taxa, with JH from the corpora allata, ecdysteroids from the prothoracic glands or the ovary, and brain neurohormones (neuro-peptides such as ovarian ecdysteroidogenic hormone, OEH) considered to induce or stimulate vitellogenin synthesis to varying degrees in different insect species (Fig. 5.13).
Inhibition of egg development in ovarian follicles in the previtellogenic stage is mediated by antigonadotropins. This inhibition ensures that only some of the oocytes undergo vitellogenesis in each ovarian cycle. The antigonadotropins responsible for this suppression are peptides termed oostatic hormones. In most of the insects studied, oostatic hormones are produced by the ovary or neurosecretory tissue associated with the ovary and, depending on species, may work in one of three ways:
- inhibit the release or synthesis of OEH (also called egg development neurohormone, EDNH); or
- affect ovarian development by inhibiting proteolytic enzyme synthesis and blood digestion in the midgut, as in mosquitoes; or
- inhibit the action of JH on vitellogenic follicle cells thus preventing the ovary from accumulating vitellogenin from the hemolymph, as in the blood-sucking bug Rhodnius prolixus.
Originally, it was firmly believed that JH controlled vitellogenesis in most insects. Then, in certain insects, the importance of ecdysteroids was discovered. Now we are becoming increasingly aware of the part played by neuropeptides, a group of proteins for which reproductive regulation is but one of an array of functions in the insect body (see Table 3.1).
Table 3.1. Examples of some important insect physiological processes mediated by neuropeptides.
(After Keeley & Hayes 1987; Holman et al. 1990; Gäde et al. 1997; Altstein 2003.)
| Neuropeptide | Action |
|---|---|
| Growth and development | |
| Allatostatins and allatotropins | Induce/regulate juvenile hormone (JH) production |
| Bursicon | Controls cuticular sclerotization |
| Crustacean cardioactive peptide (CCAP) | Switches on ecdysis behavior |
| Diapause hormone (DH) | Causes dormancy in silkworm eggs |
| Pre-ecdysis triggering hormone (PETH) | Stimulates pre-ecdysis behavior |
| Ecdysis triggering hormone (ETH) | Initiates events at ecdysis |
| Eclosion hormone (EH) | Controls events at ecdysis |
| JH esterase inducing factor | Stimulates JH degradative enzyme |
| Prothoracicotropic hormone (PTTH) | Induces ecdysteroid secretion from prothoracic gland |
| Puparium tanning factor | Accelerates fly puparium tanning |
| Reproduction | |
| Antigonadotropin (e. g. oostatic hormone, OH) | Suppresses oocyte development |
| Ovarian ecdysteroidogenic hormone (OEH = EDNH) | Stimulates ovarian ecdysteroid production |
| Ovary maturing peptide (OMP) | Stimulates egg development |
| Oviposition peptides | Stimulate egg deposition |
| Prothoracicotropic hormone (PTTH) | Affects egg development |
| Pheromone biosynthesis activating neuropeptide | Regulates pheromone production (PBAN) |
| Homeostasis | |
| Metabolic peptides (= AKH/RPCH family) | |
| Adipokinetic hormone (AKH) | Releases lipid from fat body |
| Hyperglycemic hormone | Releases carbohydrate from fat body |
| Hypoglycemic hormone | Enhances carbohydrate uptake |
| Protein synthesis factors | Enhance fat body protein synthesis |
| Diuretic and antidiuretic peptides | |
| Antidiuretic peptide (ADP) | Suppresses water excretion |
| Diuretic peptide (DP) | Enhances water excretion |
| Chloride-transport stimulating hormone | Stimulates Cl− absorption (rectum) |
| Ion-transport peptide (ITP) | Stimulates Cl− absorption (ileum) |
| Myotropic peptides | |
| Cardiopeptides | Increase heartbeat rate |
| Kinin family (e. g. leukokinins and myosuppressins) | Regulate gut contraction |
| Proctolin | Modifies excitation response of some muscles |
| Chromatotropic peptides | |
| Melanization and reddish coloration hormone (MRCH) | Induces darkening |
| Pigment-dispersing hormone (PDH) | Disperses pigment |
| Corazonin | Darkens pigment |
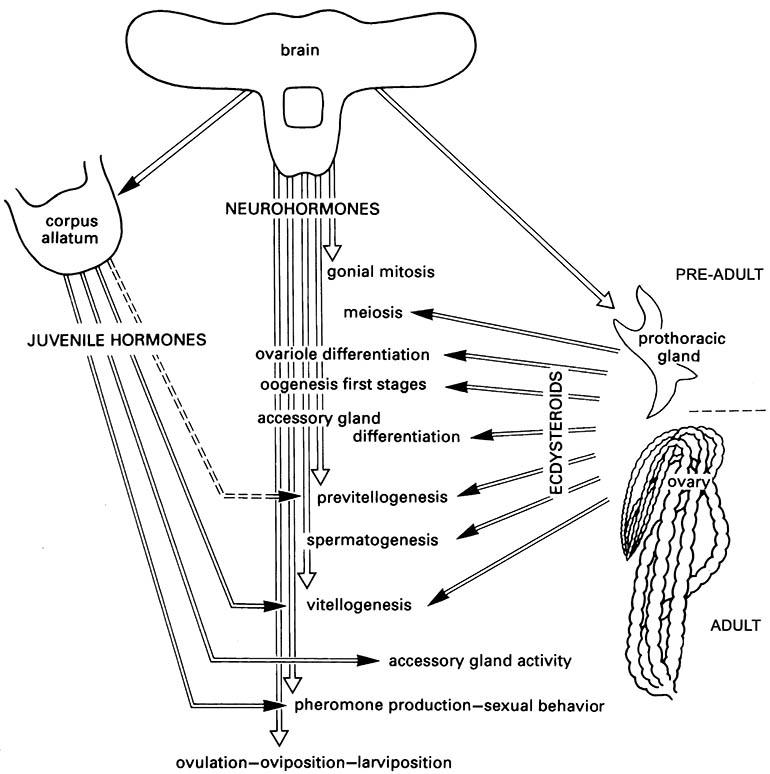
The transition from ecdysterone production by the pre-adult prothoracic gland to the adult ovary varies between taxa. (After Raabe 1986)

