12.1.2. Parental care as a social behavior
Parental care may be considered to be a social behavior; although few insects, if any, show a complete lack of parental care: eggs are not deposited randomly. Females select an appropriate oviposition site, affording protection to the eggs and ensuring an appropriate food resource for the hatching offspring. The ovipositing female may protect the eggs in an ootheca, or deposit them directly into suitable substrate with her ovipositor, or modify the environment, as in nest construction. Parental care conventionally is seen as postoviposition and/or posthatching attention, including the provision and protection of food resources for the young. A convenient basis for discussing parental care is to distinguish between care with and without nest construction.
Parental care without nesting
For most insects, the highest mortality occurs in the egg and first instar, and many insects tend these stages until the more mature larvae or nymphs can better fend for themselves. The orders of insects in which tending of eggs and young is most frequent are the Blattodea, Orthoptera, and Dermaptera (orthopteroid orders), Embiidina, Psocoptera, Thysanoptera, Hemiptera, Coleoptera, and Hymenoptera. There has been a tend- ency to assume that subsociality is a precursor of isopteran eusociality, as the eusocial termites are related to cockroaches. The phylogenetic position (Fig. 7.4) and social behavior, including parental care, of the subsocial cockroach family Cryptocercidae has provoked speculation on the origin of sociality, discussed in more detail in section 12.4.2.
Egg and early-instar attendance is predominantly a female role; yet paternal guarding is known in some Hemiptera, notably amongst some tropical assassin bugs (Reduviidae) and giant water bugs (Belostomatidae). The female belostomatid oviposits onto the dorsum of the male, which receives eggs in small batches after each copulation. The eggs, which die if neglected, are tended in various ways by the male (Box 5.5). There is no tending of belostomatid nymphs, unlike some other hemipterans in which the female (or in some reduviids, the male) may guard at least the early-instar nymphs. In these species, experimental removal of the tending adult increases losses of eggs and nymphs as a result of parasitization and/or predation. Other functions of parental care include keeping the eggs free from fungi, maintaining appropriate conditions for egg development, herding the young, and sometimes actually feeding them.
In an unusual case, certain treehoppers (Hemiptera: Membracidae) have “delegated” parental care of their young to ants. Ants obtain honeydew from treehoppers, which are protected from their natural enemies by the presence of the ants. In the presence of protective ants, brooding females prematurely may cease to tend a first brood and raise a second one. Another species of membracid will abandon its eggs in the absence of ants and seek a larger treehopper aggregation, where ants are in attendance, before laying another batch of eggs.
Many wood-mining beetles show advanced subsocial care that verges on the nesting described in the following section and on eusociality. For instance, all Passalidae (Coleoptera) live in communities of larvae and adults, with the adults chewing dead wood to form a substrate for the larvae to feed upon. Some ambrosia beetles (Curculionidae: Platypodinae) prepare galleries for their offspring (section 9.2), where the larvae feed on cultivated fungus and are defended by a male that guards the tunnel entrance. Whether or not these feeding galleries are called nests is a matter of semantics.
Parental care with solitary nesting
Nesting is a social behavior in which eggs are laid in a pre-existing or newly constructed structure to which the parent(s) bring food supplies for the young. Nesting, as thus defined, is seen in only five insect orders. Nest builders amongst the subsocial Orthoptera, Dermaptera, Coleoptera, and subsocial Hymenoptera are discussed below; the nests of eusocial Hymenoptera and the prodigious mounds of the eusocial termites are discussed later in this chapter.
Earwigs of both sexes overwinter in a nest. In spring, the male is ejected when the mother starts to tend the eggs (Fig. 9.1). In some species mother earwigs forage and provide food for the young nymphs. Mole crickets and other ground-nesting crickets exhibit somewhat similar behavior. A greater range of nesting behaviors is seen in the beetles, particularly in the dung beetles (Scarabaeidae) and carrion beetles (Silphidae). For these insects the attractiveness of the short-lived, scattered, but nutrient-rich dung (and carrion) food resource induces competition. Upon location of a fresh source, dung beetles bury it to prevent drying out or being ousted by a competitor (section 9.3; Fig. 9.5). Some scarabs roll the dung away from its source; others coat the dung with clay. Both sexes co-operate, but the female is mostly responsible for burrowing and preparation of the larval food source. Eggs are laid on the buried dung and in some species no further interest is taken. However, parental care is well developed in others, commonly with maternal attention to fungus reduction, and removal or exclusion of conspecifics and ants by paternal defense.
Amongst the Hymenoptera, subsocial nesting is restricted to some aculeate Apocrita within the super-families Chrysidoidea, Vespoidea, and Apoidea (Fig. 12.2); these wasps and bees are the most prolific and diverse nest builders amongst the insects. Excepting bees, nearly all these insects are parasitoids, in which adults attack and immobilize arthropod prey upon which the young feed. Wasps demonstrate a series of increasingly complex prey handling and nesting strategies, from using the prey’s own burrow (e.g. many Pompilidae), to building a simple burrow following prey capture (a few Sphecidae), to construction of a nest burrow before prey-capture (most Sphecidae). In bees and masarine wasps, pollen replaces arthropod prey as the food source that is collected and stored for the larvae. Nest complexity in the aculeates ranges from a single burrow provisioned with one food item for one developing egg, to linearly or radially arranged multicellular nests. The primitive nest site was probably a pre-existing burrow, with the construction medium later being soil or sand. Further specializations involved the use of plant material — stems, rotten wood, and even solid wood by carpenter bees (Xylocopini) — and free-standing constructions of chewed vegetation (Megachilinae), mud (Eumeninae), and saliva (Colletinae). A range of natural materials are used in making and sealing cells, including mud, plants, and saps, resins, and oils secreted by plants as rewards for pollination, and even the wax adorning soft scale insects. In some subsocial nesters such as mason wasps (Eumeninae), many individuals of one species may aggregate, building their nests close together.
Parental care with communal nesting
When favorable conditions for nest construction are scarce and scattered throughout the environment, communal nesting may occur. Even under apparently favorable conditions, many subsocial and all eusocial hymenopterans share nests. Communal nesting may arise if daughters nest in their natal nest, enhancing utilization of nesting resources and encouraging mutual defense against parasites. However, communal nesting in subsocial species allows “antisocial” or selfish behavior, with frequent theft or takeover of nest and prey, so that extended time defending the nest against others of the same species may be required. Furthermore, the same cues that lead the wasps and bees to communal nesting sites easily can direct specialized nest parasites to the location. Examples of communal nesting in subsocial species are known or presumed in the Sphecidae, and in bees among Halictinae, Megachilinae, and Andreninae.
After oviposition, female bees and wasps remain in their nests, often until the next generation emerges as adults. They generally guard, but they also may remove feces and generally maintain nest hygiene. The supply of provisions to the nest may be through mass provisioning, as in many communal sphecids and subsocial bees, or replenishment, as seen in the many vespid wasps that return with new prey as their larvae develop.
Subsocial aphids and thrips
Certain aphids belonging to the subfamilies Pemphiginae and Hormaphidinae (Hemiptera: Aphididae) have a sacrificial sterile soldier caste, consisting of some first- or second-instar nymphs that exhibit aggressive behavior and never develop into adults. Soldiers are pseudoscorpion-like, as a result of body sclerotization and enlarged anterior legs, and will attack intruders using either their frontal horns (anterior cuticular projections) (Fig. 12.1) or feeding stylets (mouthparts) as piercing weapons. These modified individuals may defend good feeding sites against competitors or defend their colony against predators. As the offspring are produced by parthenogenesis, soldiers and normal nymphs from the same mother aphid should be genetically identical, favoring the evolution of these non-reproductive and apparently altruistic soldiers (as a result of increased inclusive fitness via kin selection; section 12.4). A similar phenomenon occurs in other related aphid species, but in this case all nymphs become temporary soldiers, which later molt into normal, non-aggressive individuals that reproduce. These unusual aphid polymorphisms have led some researchers to claim that the Hemiptera is a third insect order displaying eusociality. Although these few aphid species clearly have a reproductive division of labor, they do not appear to fulfill the other attributes of eusocial insects, as overlap of generations capable of contributing to colony labor is equivocal and tending of offspring does not occur. Here we consider these aphids to exhibit subsocial behavior.
A range of subsocial behaviors is seen in a few species of several genera of thrips (Thysanoptera: Phlaeothripidae). At least in the gall thrips, the level of sociality appears to be similar to that of the aphids discussed above. Thrips sociality is well developed in a bark-dwelling species of Anactinothrips from Panama, in which thrips live communally, co-operate in brood care, and forage with their young in a highly co-ordinated fashion. However, this species has no obvious non- reproductive females and all adults may disappear before the young are fully grown. Evolution of subsocial behaviors in Anactinothrips may bring advantages to the young in group foraging, as feeding sites, although stable over time, are patchy and difficult to locate. In several species of Australian gall thrips, females show polymorphic wing reduction associated in some species with very enlarged fore legs. This “soldier” morph is more frequent amongst the first young to develop, which are differentially involved in defending the gall against intrusion by other species of thrips, and appear to be incapable of dispersing or of inducing galls. As in most thrips, sex determination is via haplodiploidy, with gall foundation by a single female producing polymorphic offspring, and with establishment of multiple generations. Self-sacrificing defense by some individuals is favored by demonstrated high relatedness of the offspring (altruism; section 12.4). Generational overlap is modest, and soldiers defend their siblings and their off- spring rather than their mother (who has died). Soldiers reproduce, but at a much lower rate than the foundress. Such examples are valuable in showing the circumstances under which co-operation might have evolved.
Quasisociality and semisociality
Division of reproductive labor is restricted to the subsocial aphids amongst the insect groups discussed above: all females of all the other subsocial insects can reproduce. Within the social Hymenoptera, females show variation in fecundity, or reproductive division of labor. This variation ranges from fully reproductive (the subsocial species described above), through reduced fecundity (many halictine bees), the laying of only male eggs (workers of Bombus), sterility (workers of Aphaenogaster), to super-reproductives (queens of Apis). This range of female behaviors is reflected in the classification of social behaviors in the Hymenoptera. Thus, in quasisocial behavior, a communal nest consists of members of the same generation all of which assist in brood rearing, and all females are able to lay eggs, even if not necessarily at the same time. In semisocial behavior, the communal nest similarly contains members of the same generation co-operating in brood care, but there is division of reproductive labor, with some females (queens) laying eggs, whereas their sisters act as workers and rarely lay eggs. This differs from eusociality only in that the workers are sisters to the egg-laying queens, rather than daughters, as is the case in eusociality. As in primitive eusocial hymenopterans there is no morphological (size or shape) difference between queens and workers.
Any or all the subsocial behaviors discussed above may be evolutionary precursors of eusociality. It is clear that solitary nesting is the primitive behavior, with communal nesting (and additional subsocial behaviors) having arisen independently in many lineages of aculeate hymenopterans.

The broken line indicates a paraphyletic taxon. (Data from several sources)
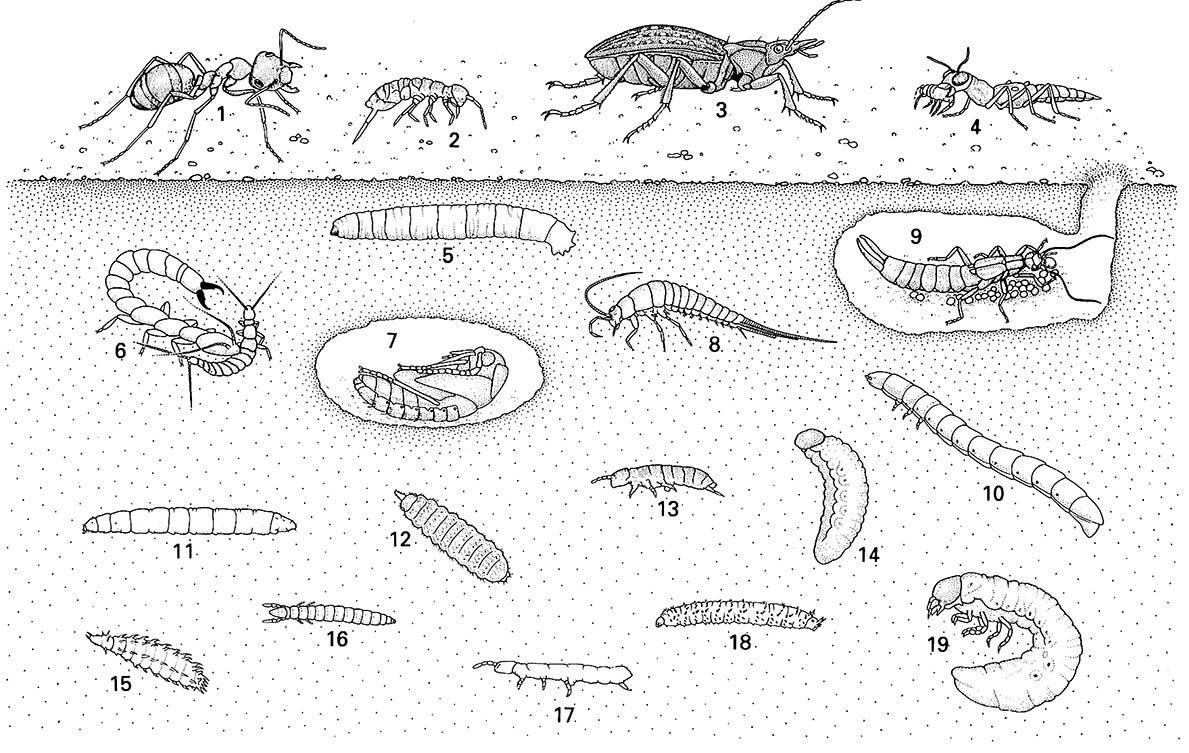
Note that organisms living on the soil surface and in litter have longer legs than those found deeper in the ground. Organisms occurring deep in th e soil usually are legless or have reduced legs; they are unpigmented and often blind. The organisms depicted are: (1) worker of a woo d ant (Hymenoptera: Formicidae); (2) springtail (Collembola: Isotomidae); (3) ground beetle (Coleoptera: Carabidae); (4) rove beetle (Coleoptera: Staphylinidae) eating a springtail; (5) larva of a crane fly (Diptera: Tipulidae); (6) japygid dipluran (Dip lura: Japygidae) attacking a smaller campodeid dipluran; (7) pupa of a ground beetle (Coleoptera: Carabidae); (8) bristletail (Archaeognatha: Machilidae); (9) female earwig (Dermaptera: Labiduridae) tending her eggs; (10) wireworm, larva of a tenebrionid beetle (Coleoptera: Tenebrionidae); (11) larva of a robber fly (Diptera: Asilidae); (12) larva of a soldier fly (Dipt era: Stratiomyidae); (13) springtail (Collembola: Isotomidae); (14) larva of a weevil (Coleoptera: Curculionidae); (15) larva of a m uscid fly (Diptera: Muscidae); (16) proturan (Protura: Sinentomidae); (17) springtail (Collembola: Isotomidae); (18) larva of a March fly (Diptera: Bibionidae); (19) larva of a scarab beetle (Coleoptera: Scarabaeidae). (Individual organisms after various sources, especially Eisenbeis & Wichard 1987)
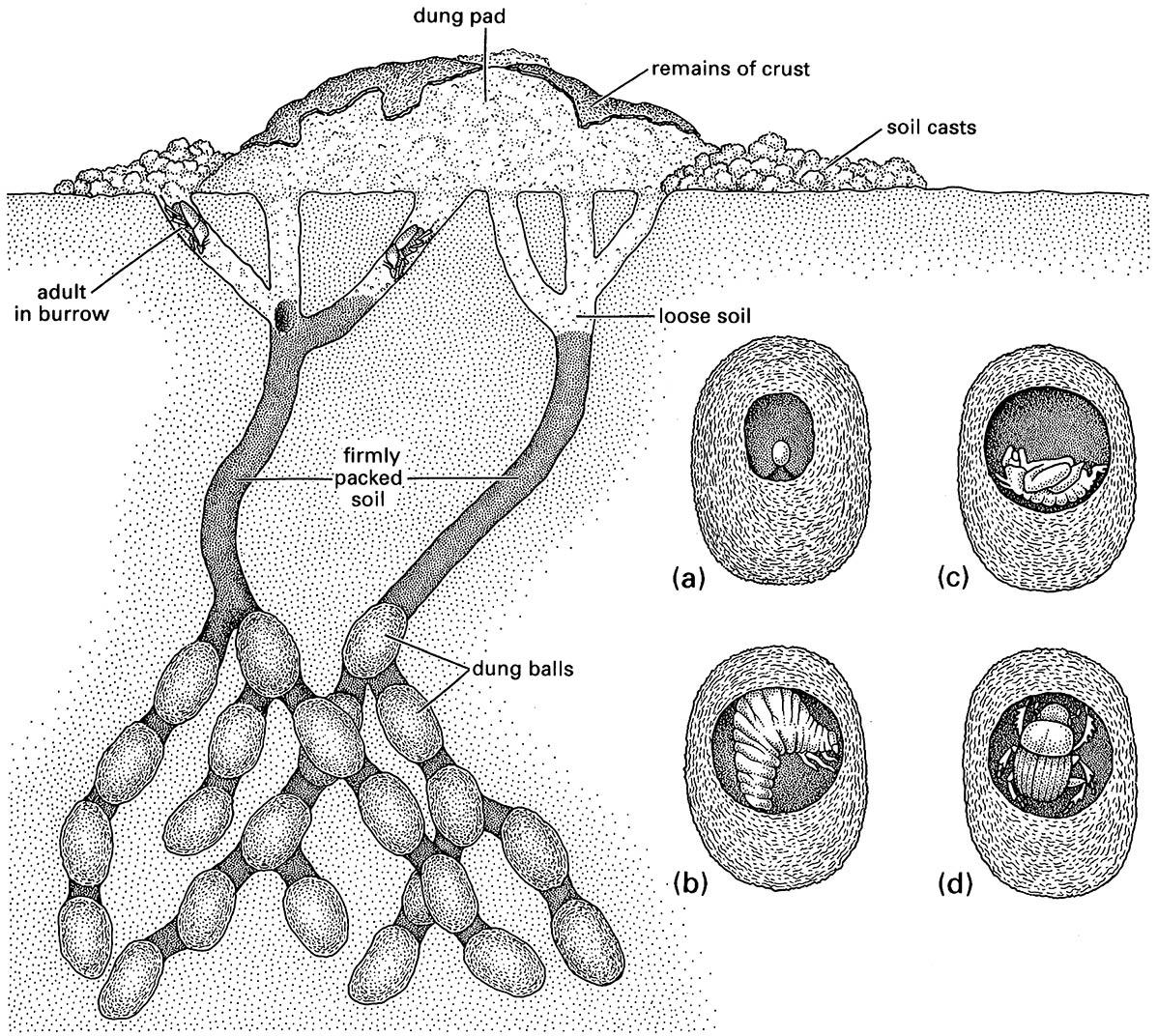
The inset shows an individual dung ball within which beetle development takes place: (a) egg; (b) larva, which feeds on the dung; (c) pupa; and (d) adult just prior to emergence. (After Waterhouse 1974)
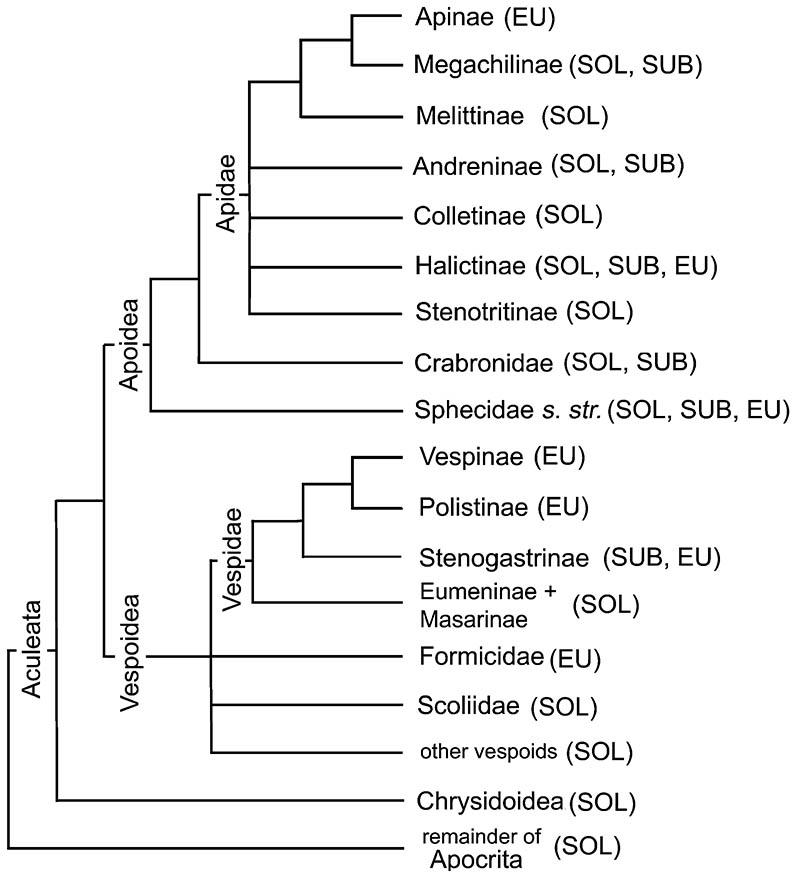
The superfamily Apoidea includes the Sphecidae sensu stricto, the Crabronidae (formerly part of a broader Sphecidae), the Ampulicidae (not shown), and all bees, here treated as one family, the Apidae, with several subfamilies (e.g. Apinae, Colletinae, Halictinae; not all solitary groups are shown) of uncertain relationships. Traditionally, bees have been classified in several families, a ranking that is unjustified phylogenetically. Probable relationships within non-social aculeate wasps (e.g. Ampulicidae, Pompilidae, and Rhopalosomatidae) and bees are not depicted. (Adapted from several sources including Gauld & Bolton 1988; Alexander 1992; Brothers 1999; B.N. Danforth, pers. comm.)
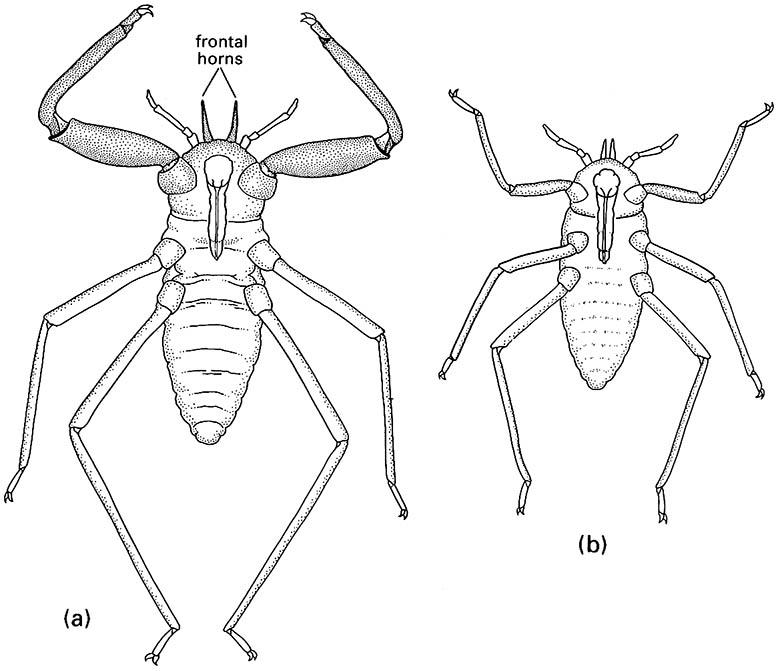
(a) pseudoscorpion-like soldier; (b) normal nymph. (After Miyazaki 1987b.)

