12.4.1. The origins of eusociality in Hymenoptera
According to estimates derived from the proposed phylogeny of the Hymenoptera, eusociality has arisen independently in wasps, bees, and ants (Fig. 12.2) with multiple origins within wasps and bees, and some losses by reversion to solitary behavior. Comparisons of life histories between living species with different degrees of social behavior allow extrapolation to possible historical pathways from solitariness to sociality. Three possible routes have been suggested and in each case, communal living is seen to provide benefits through sharing the costs of nest construction and defense of offspring.
The first suggestion envisages a monogynous (single queen) subsocial system with eusociality developing through the queen remaining associated with her offspring through increased maternal longevity.
In the second scenario, involving semisociality and perhaps applicable only to certain bees, several unrelated females of the same generation associate and establish a colonial nest in which there is some reproductive division of labor, with an association that lasts only for one generation.
The third scenario involves elements of the previous two, with a communal group comprising related females (rather than unrelated) and multiple queens (in a polygynous system), within which there is increasing reproductive division. The association of queens and daughters arises through increased longevity.
These life-history-based scenarios must be considered in relation to genetic theories concerning eusociality, notably concerning the origins and maintenance by selection of altruism (or self-sacrifice in reproduction). Ever since Darwin, there has been debate about altruism — why should some individuals (non-reproductive workers) sacrifice their reproductive potential for the benefit of others?
Four proposals for the origins of the extreme reproductive sacrifice seen in eusociality are discussed below. Three proposals are partially or completely compatible with one another, but group selection, the first considered, seems incompatible. In this case, selection is argued to operate at the level of the group: an efficient colony with an altruistic division of reproductive labor will survive and produce more offspring than one in which rampant individual self-interest leads to anarchy. Although this scenario aids in understanding the maintenance of eusociality once it is established, it contributes little if anything to explaining the origin(s) of reproductive sacrifice in non-eusocial or subsocial insects. The concept of group selection operating on pre-eusocial colonies runs counter to the view that selection operates on the genome, and hence the origin of altruistic individual sterility is difficult to accept under group selection. It is amongst the remaining three proposals, namely kin selection, maternal manipulation, and mutualism, that the origins of eusociality are more usually sought.
The first, kin selection, stems from recognition that classical or Darwinian fitness — the direct genetic contribution to the gene pool by an individual through its offspring — is only part of the contribution to an individual’s total, or inclusive, or extended, fitness. An additional indirect contribution, termed the kinship component, must be included. This is the contribution to the gene pool made by an individual that assists and enhances the reproductive success of its kin. Kin are individuals with similar or identical genotypes derived from the relatedness due to having the same parents. In the Hymenoptera, kin relatedness is enhanced by the haplodiploid genetic system. In this system, males are haploid so that each sperm (produced by mitosis) contains 100% of the paternal genes. In contrast, the egg (produced by meiosis) is diploid, containing only half the maternal genes. Thus, daughter offspring, produced from fertilized eggs, share all their father’s genes, but only half of their mother’s genes. Because of this, full sisters (i.e. those with the same father) share on average three-quarters of their genes. Therefore, sisters share more genes with each other than they would with their own female offspring (50%). Under these conditions, the inclusive fitness of a sterile female (worker) is greater than its classical fitness. As selection operating on an individual should maximize its inclusive fitness, a worker should invest in the survival of her sisters, the queen’s offspring, rather than in the production of her own female young.
However, haplodiploidy alone is an inadequate explanation for the origin of eusociality, because altruism does not arise solely from relatedness. Haplodiploidy is universal in hymenopterans and kinship has encouraged repeated eusociality, but eusociality is not universal in the Hymenoptera. Furthermore, other haplodiploid insects such as thrips are not eusocial, although there may be social behavior. Other factors promoting eusociality are recognized in Hamilton’s rule, which emphasizes the ratio of costs and benefits of altruistic behavior as well as relatedness. The conditions under which selection will favor altruism can be expressed as follows:
rB — C > 0
where r is the coefficient of relatedness, B is the benefit gained by the recipient of altruism, and C is the cost suffered by the donor of altruism. Thus, variations in benefits and costs modify the consequences of the particular degrees of relatedness expressed in Fig. 12.11, although these factors are difficult to quantify.
Kinship calculations assume that all offspring of a single mother in the colony share an identical father, and this assumption is implicit in the kinship scenario for the origin of eusociality. At least in higher eusocial insects, queens may mate multiply with different males, and thus r values are less than predicted by the monogamous model. This effect impinges on maintenance of an already existing eusocial system, discussed below in section 12.4.3. Whatever, the opportunity to help relatives, in combination with high relatedness through haplodiploidy, predisposes insects to eusociality.
At least two further ideas concern the origins of eusociality. The first involves maternal manipulation of offspring (both behaviorally and genetically), such that by reducing the reproductive potential of certain offspring, parental fitness may be maximized by assuring reproductive success of a few select offspring. Most female Aculeata can control the sex of offspring through fertilizing the egg or not, and are able to vary offspring size through the amount of food supplied, making maternal manipulation a plausible option for the origin of eusociality.
A further well-supported scenario emphasizes the roles of competition and mutualism. This envisages individuals acting to enhance their own classical fitness with contributions to the fitness of neighbors arising only incidentally. Each individual benefits from colonial life through communal defense by shared vigilance against predators and parasites. Thus, mutualism (including the benefits of shared defense and nest construction) and kinship encourage the establishment of group living. Differential reproduction within a familial-related colony confers significant fitness advantages on all members through their kinship. In conclusion, the three scenarios are not mutually exclusive, but are compatible in combination, with kin selection, female manipulation, and mutualism acting in concert to encourage evolution of eusociality.
The Vespinae illustrate a trend to eusociality commencing from a solitary existence, with nest-sharing and facultative labor division being a derived condition. Further evolution of eusocial behavior is envisaged as developing through a dominance hierarchy that arose from female manipulation and reproductive competition among the nest-sharers: the “winners” are queens and the “losers” are workers. From this point onwards, individuals act to maximize their fitness and the caste system becomes more rigid. As the queen and colony acquire greater longevity and the number of generations retained increases, short-term monogynous societies (those with a succession of queens) become long-term, monogynous, matrifilial (mother—daughter) colonies. Exceptionally, a derived polygynous condition may arise in large colonies, and/or in colonies where queen dominance is relaxed.
The evolution of sociality from solitary behavior should not be seen as unidirectional, with the eusocial bees and wasps at a “pinnacle”. Recent phylogenetic studies show many reversions from eusocial to semisocial and even to solitary lifestyles. Such reversions have occurred in halictine and allodapine bees. These losses demonstrate that even with haplodiploidy predisposing towards group living, unsuitable environmental conditions can counter this trend, with selection able to act against eusociality.
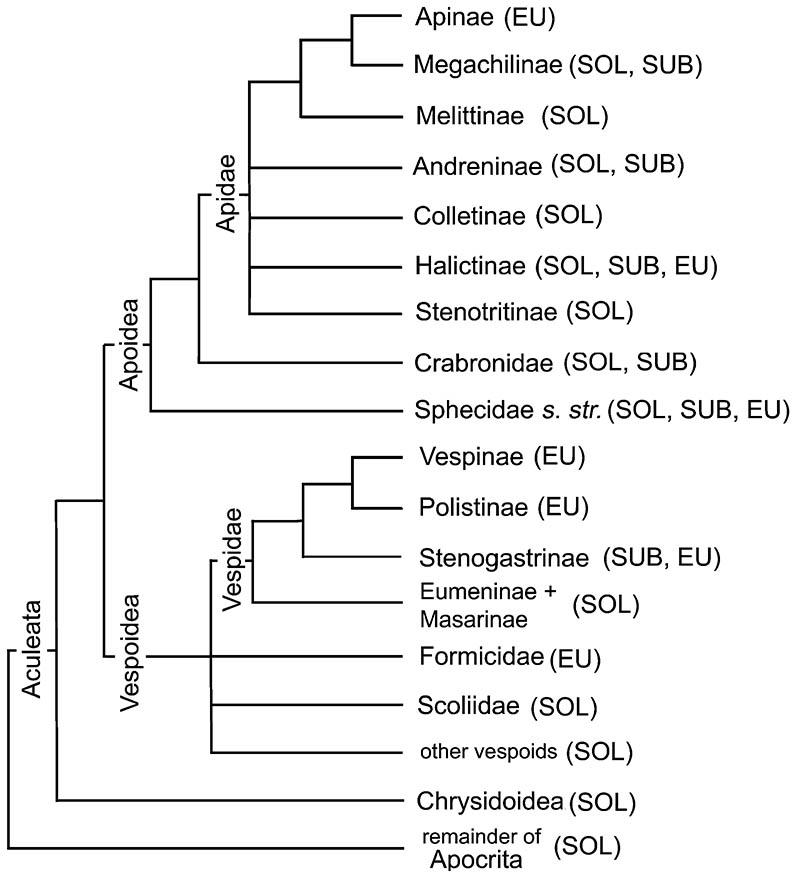
The superfamily Apoidea includes the Sphecidae sensu stricto, the Crabronidae (formerly part of a broader Sphecidae), the Ampulicidae (not shown), and all bees, here treated as one family, the Apidae, with several subfamilies (e.g. Apinae, Colletinae, Halictinae; not all solitary groups are shown) of uncertain relationships. Traditionally, bees have been classified in several families, a ranking that is unjustified phylogenetically. Probable relationships within non-social aculeate wasps (e.g. Ampulicidae, Pompilidae, and Rhopalosomatidae) and bees are not depicted. (Adapted from several sources including Gauld & Bolton 1988; Alexander 1992; Brothers 1999; B.N. Danforth, pers. comm.)

(After Whitfield 2002)

