12.2.4. Isoptera (termites)
All termites (Isoptera) are eusocial. Their diagnostic features and biology are summarized in Box 12.3.
Colony and castes in termites
In contrast to the adult and female-only castes of holometabolous eusocial Hymenoptera, the castes of the hemimetabolous Isoptera involve immature stages and equal representation of the sexes. However, before castes are discussed further, terms for termite immature stages must be clarified. Termitologists refer to the developmental instars of reproductives as nymphs, more properly called brachypterous nymphs; and the instars of sterile lineages as larvae, although strictly the latter are apterous nymphs.
The termites may be divided into two groups — the “lower” and “higher” termites. The species-rich higher termites (Termitidae) differ from lower termites in the following manners:
- Members of the Termitidae lack the symbiotic flagel-lates found in the hind gut of lower termites; these protists (protozoa) secrete enzymes (including cellulases) that may contribute to the breakdown of gut contents. One subfamily of Termitidae uses a cultivated fungus to predigest food.
- Termitidae have a more elaborate and rigid caste system. For example, in most lower termites there is little or no distension of the queen’s abdomen, whereas termitid queens undergo extraordinary physogastry, in which the abdomen is distended to 500–1000% of its original size (Fig. 12.8; see Plate 5.4).
All termite colonies contain a pair of primary reproductives — the queen and king (Plate 5.4), which are former alate (winged) adults from an established colony. Upon loss of the primary reproductives, potential replacement reproductives occur (in some species a small number may be ever-present). These individuals, called supplementary reproductives, or neotenics, are arrested in their development, either with wings present as buds (brachypterous neotenics) or without wings (apterous neotenics, or ergatoids), and can take on the reproductive role if the primary reproductives die.
In contrast to these reproductives, or potentially reproductive castes, the colony is dominated numerically by sterile termites that function as workers and soldiers of both sexes. Soldiers have distinctive heavily sclerotized heads, with large mandibles or with a strongly produced snout (or nasus) through which sticky defensive secretions are ejected. Two classes, major and minor soldiers, may occur in some species. Workers are unspecialized, weakly pigmented and poorly sclerotized, giving rise to the popular name of “white ants”.
Caste differentiation pathways are portrayed best in the more rigid system of the higher termites (Termitidae), which can then be contrasted with the greater plasticity of the lower termites. In Nasutitermes exitiosus (Termitidae: subfamily Nasutitermitinae) (Fig. 12.8), two different developmental pathways exist; one leads to reproductives and the other (which is further subdivided) gives rise to sterile castes. This differentiation may occur as early as the first larval stage, although some castes may not be recognizable morphologically until later molts. The reproductive pathway (on the left in Fig. 12.8) is relatively constant between termite taxa and typically gives rise to alates — the winged reproductives that leave the colony, mate, disperse, and found new colonies. In N. exitiosus no neotenics are formed; replacement for lost primary reproductives comes from amongst alates retained in the colony. Other Nasutitermes show great developmental plasticity.
The sterile (neuter) lineages are complex and variable between different termite species. In N. exitiosus, two categories of second-instar larvae can be recognized according to size differences probably relating to sexual dimorphism, although which sex belongs to which size category is unclear. In both lineages a subsequent molt produces a third-instar nymph of the worker caste, either small or large according to the pathway. These third-instar workers have the potential (competency) to develop into a soldier (via an intervening presoldier instar) or remain as workers through several more molts. The sterile pathway of N. exitiosus involves larger workers continuing to grow at successive molts, whereas the small worker ceases to molt beyond the fourth instar. Those that molt to become presoldiers and then soldiers develop no further.
The lower termites are more flexible, exhibiting more routes to differentiation. Lower termites have no true worker caste, but employ a functionally equivalent “child-labor” pseudergate caste composed of either nymphs whose wing buds have been eliminated (regressed) by molting or, less frequently, brachypterous nymphs or even undifferentiated larvae. Unlike the “true” workers of the higher termites, pseudergates are developmentally plastic and retain the capacity to differentiate into other castes by molting. In lower termites, differentiation of nymphs from larvae, and reproductives from pseudergates, may not be possible until a relatively late instar is reached. If there is sexual dimorphism in the sterile line, the larger workers are often male, but workers may be monomorphic. This may be through the absence of sexual dimorphism, or more rarely, because only one sex is represented. Molts in species of lower termites may give:
- morphological change within a caste;
- no morphological advance (stationary molt);
- change to a new caste (such as a pseudergate to a reproductive);
- saltation to a new morphology, missing a normal intermediate instar;
- supplementation, adding an instar to the normal route;
- reversion to an earlier morphology (such as a pseudergate from a reproductive), or a presoldier from any nymph, late-instar larva, or pseudergate.
Instar determination is impossibly difficult in the light of these molting potentialities. The only inevitability is that a presoldier must molt to a soldier.
Certain unusual termites lack soldiers. Even the universal presence of only one pair of reproductives has exceptions; multiple primary queens cohabit in some colonies of some Termitidae.
Individuals in a termite colony are derived from one pair of parents. Therefore, genetic differences existing between castes either must be sex-related or due to differential expression of the genes. Gene expression is under complex multiple and synergistic influences entailing hormones (including neurohormones), external environmental factors, and interactions between colony members. Termite colonies are very structured and have high homeostasy — caste proportions are restored rapidly after experimental or natural disturbance, by recruitment of individuals of appropriate castes and elimination of individuals excess to colony needs. Homeostasis is controlled by several pheromones that act specifically upon the corpora allata and more generally on the rest of the endocrine system. In the well-studied Kalotermes, primary reproductives inhibit differentiation of supplementary reproductives and alate nymphs. Presoldier formation is inhibited by soldiers, but stimulated through pheromones produced by reproductives.
Pheromones that inhibit reproduction are produced inside the body by reproductives and disseminated to pseudergates by proctodeal trophallaxis, i.e. by feeding on anal excretions. Transfer of pheromones to the rest of the colony is by oral trophallaxis. This was demonstrated experimentally in a Kalotermes colony by removing reproductives and dividing the colony into two halves with a membrane. Reproductives were reintroduced, orientated within the membrane such that their abdomens were directed into one half of the colony and their heads into the other. Only in the “head-end” part of the colony did pseudergates differentiate as reproductives: inhibition continued at the “abdomen-end”. Painting the protruding abdomen with varnish eliminated any cuticular chemical messengers but failed to remove the inhibition on pseudergate development. In constrast, when the anus was blocked, pseudergates became reproductive, thereby verifying anal transfer. The inhibitory pheromones produced by both queen and king have complementary or synergistic effects: a female pheromone stimulates the male to release inhibitory pheromone, whereas the male pheromone has a lesser stimulatory effect on the female. Production of primary and supplementary reproductives involves removal of these pheromonal inhibitors produced by functioning reproductives.
Increasing recognition of the role of JH in caste differentiation comes from observations such as the differentiation of pseudergates into soldiers after injection or topical application of JH or implantation of the corpora allata of reproductives. Some of the effects of pheromones on colony composition may be due to JH production by the primary reproductives. Caste determination in Termitidae originates as early as the egg, during maturation in the ovary of the queen. As the queen grows, the corpora allata undergoes hypertrophy and may attain a size 150 times greater than the gland of the alate. The JH content of eggs also varies, and it is possible that a high JH level in the egg causes differentiation to follow the sterile lineage. This route is enforced if the larvae are fed proctodeal foods (or trophic eggs) that are high in JH, whereas a low level of JH in the egg allows differentiation along the reproductive pathway. In higher and lower termites, worker and soldier differentiation from the third-instar larva is under further hormonal control, as demonstrated by the induction of individuals of these castes by JH application.
Nesting in termites
In the warmer parts of the temperate northern hemisphere, drywood termites (Kalotermitidae, especially Cryptotermes) are most familiar because of the structural damage that they cause to timber in buildings. Termites are pests of drywood and dampwood in the subtropics and tropics, but in these regions termites may be more familiar through their spectacular mound nests. In the timber pests, colony size may be no greater than a few hundred termites, whereas in the mound formers (principally species of Termitidae and some Rhinotermitidae), several million individuals may be involved. The Formosan subterranean termite (Coptotermes formosanus, Rhinotermitidae) which mostly lives in underground nests, and is a serious pest in the south-eastern USA, can form huge colonies of up to 8 million individuals.
In all cases, a new nest is founded by a male and female following the nuptial flight of alates. A small cavity is excavated into which the pair seal themselves. Copulation takes place in this royal cell, and egg-laying commences. The first offspring are workers, which are fed on regurgitated wood or other plant matter, primed with gut symbionts, until they are old enough to feed themselves and enlarge the nest. Early in the life of the colony, production is directed towards workers, with later production of soldiers to defend the colony. As the colony matures, but perhaps not until it is 5–10 years old, production of reproductives commences. This involves differentiation of alate sexual forms at the appropriate season for swarming and foundation of new colonies.
Tropical termites can use virtually all cellulose-rich food sources, above and below the ground, from grass tussocks and fungi to living and dead trees. Workers radiate from the mound, often in subterranean tunnels, less often in above-ground, pheromone-marked trails, in search of materials. In the subfamily Macrotermitinae (Termitidae), fungi are raised in combs of termite feces within the mound, and the complete culture of fungus and excreta is eaten by the colony (section 9.5.3). These fungus-tending termites form the largest termite colonies known, with estimated millions of inhabitants in some East African species.
The giant mounds of tropical termites mostly belong to species in the Termitidae. As the colony grows through production of workers, the mound is enlarged by layers of soil and termite feces until mounds as much as a century old attain massive dimensions. Diverse mound architectures characterize different termite species; for example, the “magnetic mounds” of Amitermes meridionalis in northern Australia have a narrow north—south and broad east—west orientation, and can be used like a compass (Fig. 12.9). Orientation relates to thermoregulation, as the broad face of the mound receives maximum exposure to the warming of the early and late sun, and the narrowest face presented to the high and hot midday sun. Aspect is not the only means of temperature regulation: intricate internal design, especially in fungus-farming Macrotermes species, allows circulation of air to give microclimatic control of temperature and carbon dioxide (Fig. 12.10).
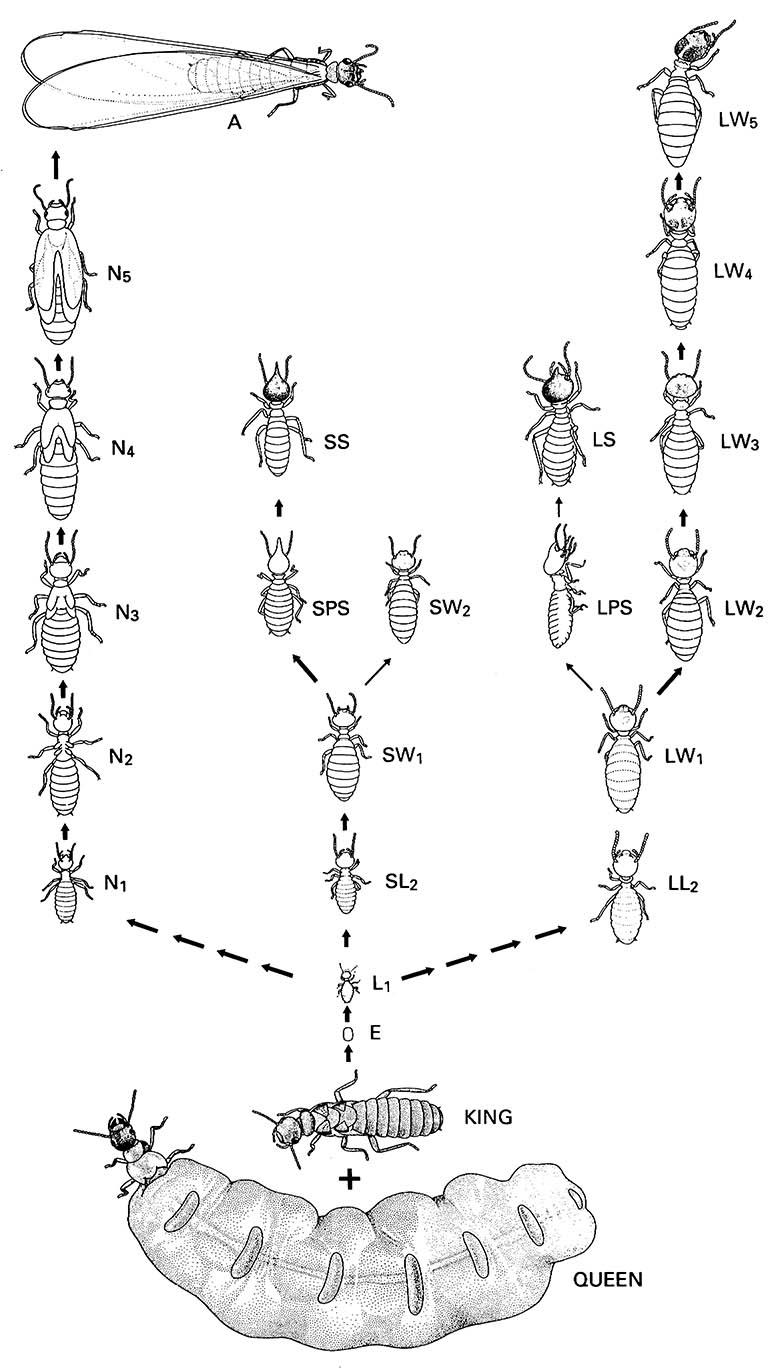
Heavy arrows indicate the main lines of development, light arrows the minor lines. A, alate; E, egg; L, larva; LL, large larva; LPS, large presoldier; LS, large soldier; LW, large worker; N, nymph; SL, small larva; SPS, small presoldier; SS, small soldier; SW, small worker. The numbers indicate the stages. (Pathways based on Watson & Abbey 1985)
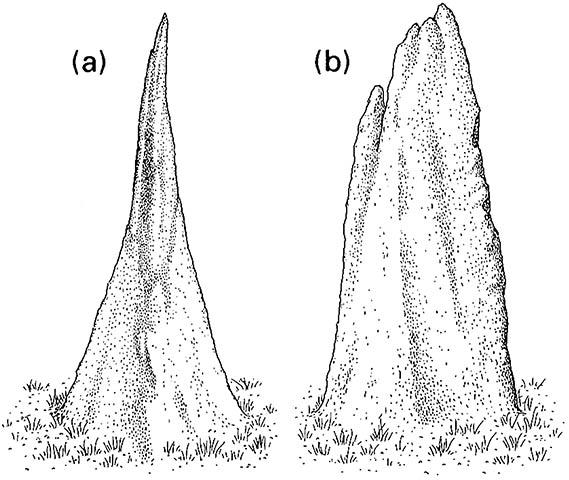
(a) the north-south view, and (b) the east-west view. (After Hadlington 1987)
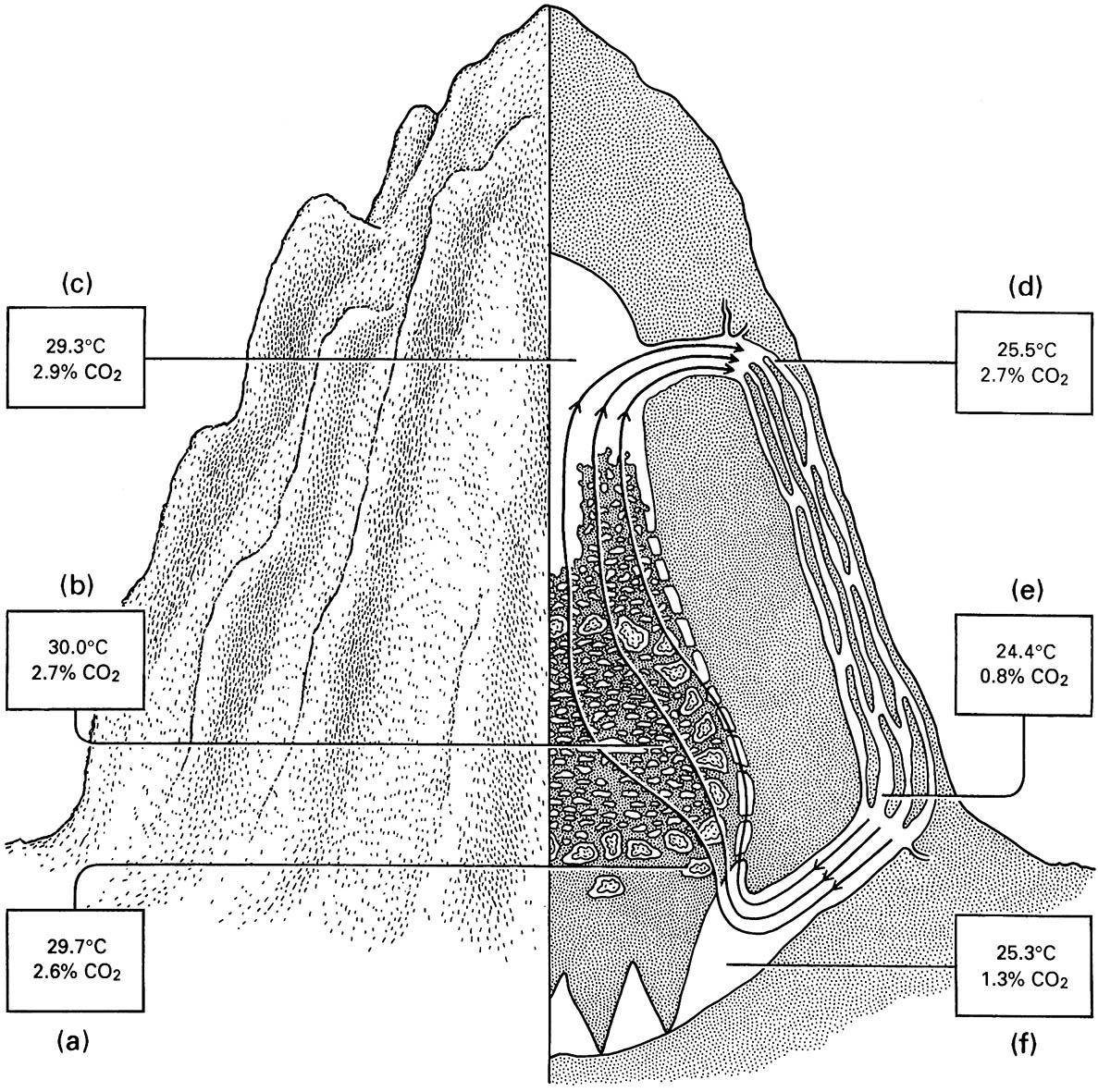
Measurements of temperature and carbon dioxide are shown in the boxes for the following locations: (a) the fungus combs; (b) the brood chambers; (c) the attic; (d) the upper part of a ridge channel; (e) the lower pa rt of a ridge channel; and (f ) the cellar. (After Lüscher 1961)

