12.3. Inquilines and parasites of social insects
The abodes of social insects provide many other insects with a hospitable place for their development. The term inquiline refers to an organism that shares a home of another. This covers a vast range of organisms that have some kind of obligate relationship with another organism, in this case a social insect. Complex classification schemes involve categorization of the insect host and the known or presumed ecological relationship between inquiline and host (e.g. myrmecophile, termitoxene). However, two alternative divisions appropriate to this discussion involve the degree of integration of the inquiline lifestyle with that of the host. Thus, integrated inquilines are incorporated into their hosts’ social lives by behavioral modification of both parties, whereas non-integrated inquilines are adapted ecologically to the nest, but do not interact socially with the host. Predatory inquilines may negatively affect the host, whereas other inquilines may merely shelter within the nest, or give benefit, such as by feeding on nest debris.
Integration may be achieved by mimicking the chemical cues used by the host in social communication (such as pheromones), or by tactile signaling that releases social behavioral responses, or both. The term Wasmannian mimicry covers some or all chemical or tactile mimetic features that allow the mimic to be accepted by a social insect, but the distinction from other forms of mimicry (notably Batesian; section 14.5.1) is unclear. Wasmannian mimicry may, but need not, include imitation of the body form. Conversely, mimicry of a social insect may not imply inquilinism — the ant mimics shown in Fig. 14.12 may gain some protection from their natural enemies as a result of their ant-like appearance, but are not symbionts or nest associates.
The breaking of the social insect chemical code occurs through the ability of an inquiline to produce appeasement and/or adoption chemicals — the messengers that social insects use to recognize one another and to distinguish themselves from intruders. Caterpillars of Maculinea arion (the large blue butterfly) and congeners that develop in the nests of red ants (Myrmica spp.) as inquilines or parasites evidently surmount the nest defenses (Box 1.1). Certain staphylinid beetles also can do this, for example Atemeles pubicollis, which lives as a larva in the nest of the European ant, Formica rufa. The staphylinid larva produces a glandular secretion that induces brood-tending ants to groom the alien. Food is obtained by adoption of the begging posture of an ant larva, in which the larva rears up and contacts the adult ant mouthparts, provoking a release of regurgitated food. The diet of the staphylinid is supplemented by predation on larvae of ants and of their own species. Pupation and adult eclosion take place in the Formica rufa nest. However, this ant ceases activity in winter and during this period the staphylinid seeks alternative shelter. Adult beetles leave the wooded Formica habitat and migrate to the more open grassland habitat of Myrmica ants. When a Myrmica ant is encountered, secretions from the “appeasement glands” are offered that suppress the aggression of the ant, and then the products of glands on the lateral abdomen attract the ant. Feeding on these secretions appears to facilitate “adoption”, as the ant subsequently carries the beetle back to its nest where the immature adult overwinters as a tolerated food-thief. In spring, the reproductively mature adult beetle departs for the woods to seek out another Formica nest for oviposition.
Amongst the inquilines of termites, many show convergence in shape in terms of physogastry (dilation of the abdomen), seen also in queen termites. In the curious case of flies of Termitoxenia and relatives (Diptera: Phoridae), the physogastric females from termite nests were the only stage known for so long that published speculation was rife that neither larvae nor males existed. It was suggested that the females hatched directly from huge eggs, were brachypterous throughout their lives (hitching a ride on termites for dispersal), and, uniquely amongst the endopterygotes, the flies were believed to be protandrous hermaphrodites, functioning first as males, then as females. The truth is more prosaic: sexual dimorphism in the group is so great that wild-caught, flying males had been unrecognized and placed in a different taxonomic group. The females are winged, but shed all but the stumps of the anterior veins after mating, before entering the termitarium. Although the eggs are large, short-lived larval stages exist. As the postmated female is stenogastrous (with a small abdomen), physogastry must develop whilst in the termitarium. Thus, Termitoxenia is only a rather unconventional fly, well adapted to the rigors of life in a termite nest, in which its eggs are treated by the termites as their own, and with attenuation of the vulnerable larval stage, rather than the possessor of a unique suite of life-history features.
Inquilinism is not restricted to non-social insects that breach the defenses (section 12.4.3) and abuse the hospitality of social insects. Even amongst the social Hymenoptera some ants may live as temporary or even permanent social parasites in the nests of other species. A reproductive female inquiline gains access to a host nest and usually kills the resident queen. In some cases, the intruder queen produces workers, which eventually take over the nest. In others, the inquiline usurper produces only males and reproductives — the worker caste is eliminated and the nest survives only until the workers of the host species die off.
In a further twist of the complex social lives of ants, some species are slave-makers; they capture pupae from the nests of other species and take them to their own nest where they are reared as slave workers. This phenomenon, known as dulosis, occurs in several inquiline species, all of which found their colonies by parasitism.
The phylogenetic relationships between ant hosts and ant inquilines reveal an unexpectedly high pro- portion of instances in which host and inquiline belong to sister species (i.e. each other’s closest relatives), and many more are congeneric close relatives. One possible explanation envisages the situation in which daughter species formed in isolation come into secondary contact after mating barriers have developed. If no differentiation of colony-identifying chemicals has taken place, it is possible for one species to invade the colony of the other undetected, and parasitization is facilitated.
Non-integrated inquilines are exemplified by hover flies of the genus Volucella (Diptera: Syrphidae), the adults of which are Batesian mimics of either Polistes wasps or of Bombus bees. Female flies appear free to fly in and out of hymenopteran nests, and lay eggs whilst walking over the comb. Hatching larvae drop to the bottom of the nest where they scavenge on fallen detritus and fallen prey. Another syrphid, Microdon, has a myrmecophilous larva so curious that it was described first as a mollusk, then as a coccoid. It lives unscathed amongst nest debris (and perhaps sometimes as a predator on young ant larvae), but the emerged adult is recognized as an intruder. Non-integrated inquilines include many predators and parasitoids whose means of circumventing the defenses of social insects are largely unknown.
Social insects also support a few parasitic arthropods. For example, varroa and tracheal mites (Acari) and the bee louse, Braula coeca (Diptera: Braulidae; section 13.3.3), all live on honey bees (Apidae: Apis spp.). The extent of colony damage caused by the tracheal mite Acarapis woodi is controversial, but infestations of Varroa are resulting in serious declines in honey-bee populations in most parts of the world. Varroa mites feed externally on the bee brood (see Plate 5.5) leading to deformation and death of the bees. Low levels of mite infestation are difficult to detect and it can take several years for a mite population to build to a level that causes extensive damage to the hive. Some Apis species, such as A. cerana, appear more resistant to varroa but interpretation is complicated by the existence of a sibling species complex of varroa mites with distinct biogeographic and virulence patterning. This suggests that great care should be taken to avoid promiscuous mixing of different bee and mite genotypes.
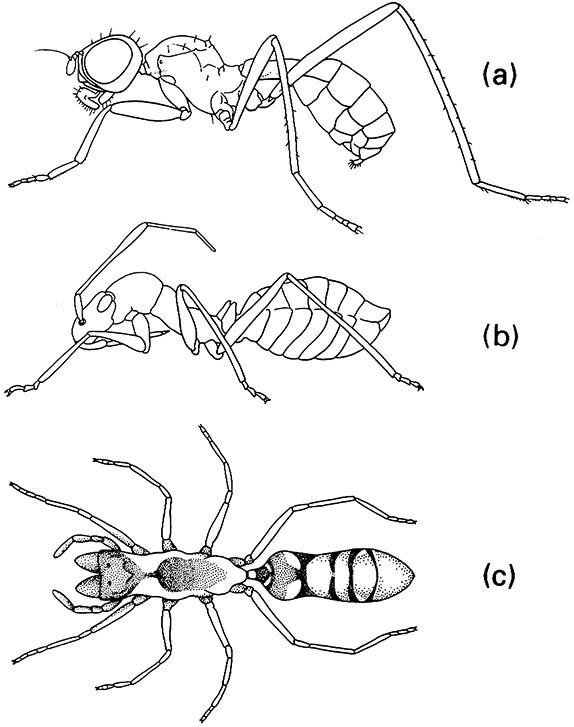
(a) a fly (Diptera: Micropezidae: Badisis); (b) a bug (Hemiptera: Miridae: Phylinae); (c) a spider (Araneae: Clubionidae: Sphecotypus). ((a) After McAlpine 1990; (b) after Atkins 1980; (c) after Oliveira 1988)

