7.4.2. Pterygota
Pterygota, treated as an infraclass, are the winged or secondarily wingless (apterous) insects, with thoracic segments of adults usually large and with the meso- and metathorax variably united to form a pterothorax. The lateral regions of the thorax are well developed. Abdominal segments number 11 or fewer, and lack styles and vesicular appendages like those of apterygotes. Most Ephemeroptera have a median terminal filament. The spiracles primarily have a muscular closing apparatus. Mating is by copulation. Metamorphosis is hemi- to holometabolous, with no adult ecdysis, except for the subimago (subadult) stage in Ephemeroptera.
Informal grouping “Palaeoptera”
Insect wings that cannot be folded against the body at rest, because articulation is via axillary plates that are fused with veins, have been termed “palaeopteran” (old wings). Living orders with such wings typically have triadic veins (paired main veins with intercalated longitudinal veins of opposite convexity/concavity to the adjacent main veins) and a network of cross-veins (figured in Boxes 10.1 and 10.2). This wing venation and articulation, together with paleontological studies of similar features, was taken to imply that Odonata and Ephemeroptera form a monophyletic group, termed Palaeoptera. The group was argued to be sister to Neoptera which comprises all remaining extant and primarily winged orders. However, reassessment of morphology of extant early-branching lineages and recent nucleotide sequence evidence fails to provide strong support for monophyly of Palaeoptera. Here we treat Ephemeroptera as sister group to Odonata + Neoptera, giving a higher classification of Pterygota into three divisions.
Division (and order) Ephemeroptera (mayflies) (see also Box 10.1)
Ephemeroptera has a fossil record dating back to the Carboniferous and is represented today by a few thousand species. In addition to their “palaeopteran” wing features mayflies display a number of unique characteristics including the non-functional, strongly reduced adult mouthparts, the presence of just one axillary plate in the wing articulation, a hypertrophied costal brace, and male fore legs modified for grasping the female during copulatory flight. Retention of a subimago (subadult stage) is unique. Nymphs (larvae) are aquatic and the mandible articulation, which is intermediate between monocondyly and the dicondylous ball-and-socket joint of all higher Insecta, may be diagnostic. Historic contraction of ephemeropteran diversity and remnant high levels of homoplasy render phylogenetic reconstruction difficult. Ephemeroptera traditionally has been divided into two suborders: Schistonota (with nymphal fore-wing pads separate from each other for over half their length) containing superfamilies Baetoidea, Heptagenioidea, Leptophlebioidea, and Ephemeroidea, and Pannota (“fused back” — with more extensively fused fore-wing pads) containing Ephemerelloidea and Caenoidea. Recent studies suggest this concept of Schistonota is paraphyletic, but no robust alternative scheme has been proposed.
Division (and order) Odonata (dragonflies and damselflies) (see also Box 10.2)
Odonates have “palaeopteran” wings as well as many additional unique features, including the presence of two axillary plates (humeral and posterior axillary) in the wing articulation and many features associated with specialized copulatory behavior, including possession of secondary copulatory apparatus on ventral segments 2–3 of the male and the formation of a tandem wheel during copulation (Box 5.3). The immature stages are aquatic and possess a highly modified prehensile labium for catching prey (Fig. 13.4).
Odonatologists (those that study odonates) traditionally recognized three groups generally ranked as suborders: Zygoptera (damselflies), Anisozygoptera and Anisoptera (dragonflies). Anisozygoptera is minor, containing fossil taxa but only one extant genus with two species. Assessment of the monophyly or paraphyly of each suborder has relied very much on interpretation of the very complex wing venation. Interpretation of wing venation within the odonates and between them and other insects has been prejudiced by prior ideas about relationships. Thus the Comstock and Needham naming system for wing veins implies that the common ancestor of modern Odonata was anisopteran, and the venation of zygopterans is reduced. In contrast, the Tillyard-named venational system implies that Zygoptera is a grade (is paraphyletic) to Anisozygoptera, which itself is a grade on the way to a monophyletic Anisoptera. A well-supported view, incorporating information from the substantial fossil record, has Zygoptera probably paraphyletic, Anisozygoptera undoubtedly paraphyletic, and Anisoptera as monophyletic sister to some extinct anisozygopterans.
Zygoptera contains three broad superfamilial groupings, the Coenagrionoidea, Lestoidea, and Calopterygoidea. Amongst Anisoptera four major lineages can be recognized, but their relationships to each other are obscure.
Division Neoptera Neopteran (“new wing”) insects diagnostically have wings capable of being folded back against their abdomen when at rest, with wing articulation that derives from separate movable sclerites in the wing base, and wing venation with none to few triadic veins and mostly lacking anastomosing (joining) cross-veins (Fig. 2.21).
The phylogeny (and hence classification) of the neopteran orders remains subject to debate, mainly concerning (a) the placement of many extinct orders described only from fossils of variably adequate preservation, (b) the relationships among the Polyneoptera (orthopteroid plus plecopteroid orders), and (c) the relationships of the highly derived Strepsiptera.
Here we summarize the most recent research findings, based on both morphology and molecules. No single or combined data set provides unambiguous resolution of insect order-level phylogeny and there are several areas of controversy. Some questions arise from inadequate data (insufficient or inappropriate taxon sampling) and character conflict within existing data (support for more than one relationship). In the absence of a robust phylogeny, ranking is somewhat subjective and “informal” ranks abound.
A group of 11 orders is termed the Polyneoptera (if monophyletic and considered to be sister to the remaining Neoptera) or Orthopteroid—Plecopteroid assemblage (if monophyly is uncertain). The remain- ing neopterans can be divided readily into two monophyletic groups, namely Paraneoptera (hemipteroid assemblage) and Endopterygota (= Holometabola). These three clades may be given the rank of subdivision. Polyneoptera and Paraneoptera both have plesiomorphic hemimetabolous development in contrast to the complete metamorphosis of Endopterygota.
Subdivision Polyneoptera (or Orthopteroid— Plecopteroid assemblage)
This grouping comprises the orders Plecoptera, Mantodea, Blattodea, Isoptera, Grylloblattodea, Mantophasmatodea, Orthoptera, Phasmatodea, Embiidina, Dermaptera, and Zoraptera.
Some early-branching events amongst the neopteran orders are becoming better understood, but some relationships remain poorly resolved, and often contradictory between those suggested by morphology and those from molecular data. The 11 included orders may form a monophyletic Polyneoptera based on the shared presence of tarsal plantulae (lacking only in Zoraptera) and certain analyses of nucleotide sequences. Within Polyneoptera, the grouping comprising Blattodea (cockroaches), Isoptera (termites), and Mantodea (mantids) — the Dictyoptera (Fig. 7.4) — is robust. All three orders within Dictyoptera share distinctive features of the head skeleton (perforated tentorium), mouthparts (paraglossal musculature), digestive system (toothed proventriculus), and female genitalia (shortened ovipositor above a large subgenital plate) which demonstrate monophyly substantiated by nearly all analyses based on nucleotide sequences. Dermaptera (the earwigs) and Zoraptera (zorapterans) form an unexpected higher clade based on recent nucleotide sequence data: some analyses place this group outside the Polyneoptera as sister to the remaining Neoptera, but the position is best represented as unresolved at the base of the assemblage (Fig. 7.2). The Grylloblattodea (the ice crawlers or rock crawlers; now apterous, but with winged fossils) forms a well- supported clade with the newly established order Mantophasmatodea.
Some data suggested that Orthoptera (crickets, katydids, grasshoppers, locusts, etc.), Phasmatodea (stick-insects or phasmids), and Embiidina (webspinners) may be closely related in a grouping called Orthop- teroidea, although recent investigations suggest an earlier-branching position for Orthoptera. The relationships of Plecoptera (stoneflies) to other groupings are poorly understood.
Order Plecoptera (stoneflies) (see also Box 10.3) Plecoptera are mandibulate in the adult, with filiform antennae, bulging compound eyes, two to three ocelli and subequal thoracic segments. The fore and hind wings are membranous and similar except that the hind wings are broader; aptery and brachyptery are frequent. The abdomen is 10-segmented, with remnants of segments 11 and 12 present, including cerci. Nymphs are aquatic.
Monophyly of the order is supported by few morphological characters, including in the adult the looping and partial fusion of gonads and male seminal vesicles, and the absence of an ovipositor. In nymphs the presence of strong, oblique, ventro-longitudinal muscles running intersegmentally allowing lateral undulating swimming, and the probably widespread “cercus heart”, an accessory circulatory organ associated with posterior abdominal gills, support the monophyly of the order. Nymphal plecopteran gills may occur on almost any part of the body, or may be absent. This varied distribution causes problems of homology of gills between families, and between those of Plecoptera and other orders. Whether Plecoptera are ancestrally aquatic or terrestrial is debatable. The phylogenetic position of Plecoptera is certainly amongst “lower Neoptera”, early in the diversification of the assemblage, possibly as sister group to the remainder of Polyneoptera, but portrayed here as unresolved (Fig. 7.2).
Internal relationships have been proposed as two predominantly vicariant suborders, the austral (southern hemisphere) Antarctoperlaria and northern Arctoperlaria. The monophyly of Antarctoperlaria is argued based on the unique sternal depressor muscle of the fore trochanter, lack of the usual tergal depressor, and presence of floriform chloride cells which may have a sensory function. Some included taxa are the large-sized Eustheniidae and Diamphipnoidae, the Gripopterygidae, and Austroperlidae — all southern hemisphere families. Some nucleotide sequence studies support this clade.
The sister group Arctoperlaria lacks defining morphology, but is united by a variety of mechanisms associated with drumming (sound production) associated with mate-finding. Component families Scopuridae, Taeniopterygidae, Capniidae, Leuctridae, and Nemouridae (including Notonemouridae) are essentially northern hemisphere with a lesser radiation of Notonemouridae into the southern hemisphere. Some nucleotide sequence analyses suggest paraphyly of Arctoperlaria, with most elements of Notonemouridae forming the sister group to the remainder of the families. Relationships amongst extant Plecoptera have been used in hypothesizing origins of wings from “thoracic gills”, and in tracing the possible development of aerial flight from surface flapping with legs trailing on the water surface, and forms of gliding. Current views of the phylogeny suggest these traits are secondary and reductional.
Order Isoptera (termites, white ants) (see also Box 12.3) Isoptera forms a small order of eusocial insects with a polymorphic caste system of reproductives, workers, and soldiers. Mouthparts are blattoid and mandibulate. Antennae are long and multisegmented. The fore and hind wings generally are similar, membranous, and with restricted venation; but Mastotermes (Mastotermitidae) with complex wing venation and a broad hind-wing anal lobe is exceptional. The male external genitalia are weakly developed and symmetrical, in contrast to the complex, asymmetrical genitalia of Blattodea and Mantodea. Female Mastotermes have a reduced blattoid-type ovipositor.
The Isoptera has always been considered to belong in Dictyoptera close to Blattodea, but precise relationships have been uncertain. A long-held view that Mastotermitidae is the earliest extant branch in the Isoptera is upheld by all studies — the distinctive features mentioned above evidently are plesiomorphies. Recent studies that included structure of the proventriculus and nucleotide sequence data suggest that termites arose from within the cockroaches, thereby rendering Blattodea paraphyletic (Fig. 7.4). Under this scenario, the (wingless) woodroaches of North America and eastern Asia (genus Cryptocercus) are sister group to Isoptera. Alternative suggestions of the independent origin (hence convergence) of the semisociality (parental care and transfer of symbiotic gut flagellates between generations) of Cryptocercus and the sociality of termites (section 12.4.2) no longer seem likely.
Order Blattodea (cockroaches) (see also Box 9.8) Cockroaches are dorsoventrally flattened insects with filiform, multisegmented antennae and mandibulate, ventrally projecting mouthparts. The prothorax has an enlarged, shield-like pronotum, that often covers the head; the meso- and metathorax are rectangular and subequal. The fore wings are sclerotized tegmina protecting membranous hind wings folded fan-like beneath. Hind wings often may be reduced or absent, and if present characteristically have many vein branches and a large anal lobe. The legs may be spiny and the tarsi are five-segmented. The abdomen has 10 visible segments, with a subgenital plate (sternum 9), bearing in the male well-developed asymmetrical genitalia, with one or two styles, and concealing the reduced 11th segment. Cerci have one or usually many segments; the female ovipositor valves are small, concealed beneath tergum 10.
Although long considered an order (and hence monophyletic) convincing evidence shows the termites arose from within the cockroaches, and the “order” thus is rendered paraphyletic. The sister group of the Isoptera appears to be Cryptocercus, undoubtedly a cockroach (Fig. 7.4). Other internal relationships of the Blattodea are not well understood, with apparent conflict between morphology and limited molecular data. Usually from five to eight families are recognized. Blatellidae and Blaberidae (the largest families) are thought to be sister groups. The many early fossils allocated to Blattodea that possess a well-developed ovipositor are considered best as belonging to a blattoid stemgroup, that is, from prior to the ordinal diversification of the Dictyoptera.
Order Mantodea (mantids) (see also Box 13.2) Mantodea are predatory, with males generally smaller than females. The small, triangular head is mobile, with slender antennae, large, widely separated eyes and mandibulate mouthparts. The prothorax is narrow and elongate, with the meso- and metathorax shorter. The fore wings form leathery tegmina with a reduced anal area; the hind wings are broad and membranous, with long unbranched veins and many cross-veins, but often are reduced or absent. The fore legs are raptorial, whereas the mid and hind legs are elongate for walking. The abdomen has a visible 10th segment, bearing variably segmented cerci. The ovipositor predominantly is internal and the external male genitalia are asymmetrical.
Mantodea forms the sister group to Blattodea + Isoptera (Fig. 7.4), and shares many features with Blattodea such as strong direct flight muscles and weak indirect (longitudinal) flight muscles, asymmetrical male genitalia and multisegmented cerci. Derived features of Mantodea relative to Blattodea involve modifications associated with predation, including leg morphology, an elongate prothorax, and features associated with visual predation, namely the mobile head with large, separated eyes. Internal relationships of the eight families of Mantodea are uncertain and little studied.
Order Grylloblattodea (= Grylloblattaria, Notoptera) (grylloblattids, ice crawlers or rock crawlers) (see also Box 9.4)
Grylloblattids are moderate-sized, soft-bodied insects with anteriorly projecting mandibulate mouthparts and the compound eyes are either reduced or absent. The antennae are multisegmented and the mouthparts mandibulate. The quadrate prothorax is larger than the meso- or metathorax, and wings are absent. The legs have large coxae and five-segmented tarsi. Ten abdominal segments are visible with rudiments of segment 11, including five- to nine-segmented cerci. The female has a short ovipositor, and the male genitalia are asymmetrical.
Several ordinal names have been used for these insects but Grylloblattodea is preferred because this name has the widest usage in published work and its ending matches the names of some related orders. Most of the rules of nomenclature do not apply to names above the family group and thus there is no name priority at ordinal level. The phylogenetic placement of Grylloblattodea also has been controversial, generally being argued to be relictual, either “bridging the cockroaches and orthopterans”, or “primitive amongst orthopteroids”. The antennal musculature resembles that of mantids and embiids, mandibular musculature resembles Dictyoptera, and the maxillary muscles those of Dermaptera. Embryologically grylloblattids are confirmed as orthopteroids. Molecular phylogenetic study emphasizing grylloblattids strongly supports a sister-group relationship to the newly discovered Mantophasmatodea, and these combined are sister to Dictyoptera.
Order Mantophasmatodea (see also Box 13.3) Mantophasmatodea is the most recently recognized order, comprising three families from Africa, and Baltic amber specimens. Mantophasmatodeans all are apterous, without even wing rudiments. The head is hypognathous with generalized mouthparts and long, slender, multisegmented antennae. Coxae are not enlarged, the fore and mid femora are broadened and have bristles or spines ventrally; hind legs are elongate; tarsi are five-segmented, with euplanulae on the basal four; the ariolum is very large and the distal tarsomere is held off the substrate. Male cerci are prominent, clasping and not differentially articulated with tergite 10; female cerci are short and one-segmented. A distinct short ovipositor projects beyond a short subgenital lobe, lacking any protective operculum (plate below ovipositor) as seen in phasmids. Based on morphology, placement of the new order was difficult, but relationships with phasmids (Phasmatodea) and/or ice crawlers (Grylloblattodea) were suggested. Nucleotide sequencing data have justified the rank of order, and strongly confirmed a sister-group relationship to Grylloblattodea. This grouping may be the extant remnants of radiation in the distant geological past represented by fossil taxa such as Titanoptera, Calo- neuridea, and Cnemidolestodea (perhaps an earlier name for Mantophasmatodea).
Order Orthoptera (grasshoppers, locusts, katydids, crickets) (see also Box 11.5)
Orthopterans are medium-sized to large insects with hind legs enlarged for jumping (saltation). The compound eyes are well developed, the antennae are elongate and multisegmented, and the prothorax is large with a shield-like pronotum curving downwards laterally. The fore wings form narrow, leathery tegmina, and the hind wings are broad, with numerous longitudinal and cross-veins, folded beneath the tegmina by pleating; aptery and brachyptery are frequent. The abdomen has eight or nine annular visible segments, with the two or three terminal segments reduced, and one-segmented cerci. The ovipositor is well developed, formed from highly modified abdominal appendages.
Virtually all morphological evidence and some molecular data suggested that the Orthoptera were closely related to Phasmatodea, to the extent that some entomologists united the orders. However, different wing bud development, egg morphology, and lack of auditory organs in phasmatids suggest distinction. Recent intensive molecular data place the Orthoptera as an early branch in the assemblage as shown in Fig. 7.2, but this requires further study.
The division of Orthoptera into two monophyletic suborders, Caelifera (grasshoppers and locusts — predominantly day-active, fast-moving, visually acute, terrestrial herbivores) and Ensifera (katydids and crickets — often night-active, camouflaged or mimetic, predators, omnivores, or phytophages), is supported on morphological and molecular evidence. Grylloidea probably form the sister group to all other ensiferan taxa but they are highly divergent. On grounds of some molecular and morphological data, Tettigoniidae and Haglidae form a monophyletic group, sister to Stenopelmatidae and relatives (Mormon crickets, wetas, Cooloola monsters, and the like), but alternative analyses suggest different or unresolved relationships. For Caelifera a well-supported recent proposal for four superfamilies, namely (Tridactyloidea (Tetragoidea (Eumastacoidea + “higher Caelifera”))) reconciles molecular evidence with certain earlier suggestions from morphology. The major grouping of acridoid grasshoppers (Acridoidea) lies in the unnamed clade “higher Caelifera”, which contains also several less- speciose superfamilies.
Order Phasmatodea (phasmatids, phasmids, stick-insects or walking sticks) (see also Box 11.6)
Phasmatodea exhibit body shapes that are variations on elongate cylindrical and stick-like or flattened, or often leaf-like. The mouthparts are mandibulate. The compound eyes are relatively small and placed anterolaterally, with ocelli only in winged species, and often only in males. The wings, if present, are functional in males, but often reduced in females, and many species are apterous in both sexes. Fore wings form short leathery tegmina, whereas the hind wings are broad with a network of numerous cross-veins and with the anterior margin toughened to protect the folded wing. The legs are elongate, slender, and adapted for walking, with five-segmented tarsi. The abdomen is 11-segmented, with segment 11 often forming a concealed supra-anal plate in males or a more obvious segment in females.
Phasmatodea have long been considered as sister to Orthoptera within the orthopteroid assemblage. Recent evidence from morphology in support of this grouping comes from neurophysiological studies, namely the dorsal position of the cell body of salivary neuron 1 in the suboesophageal ganglion and presence of serotonin in salivary neuron 2. Phasmatodea are distinguished from the Orthoptera by their body shape, asymmetrical male genitalia, proventricular structure, and lack of rotation of nymphal wing pads during development. Recent evidence for a sister-group relationship to Embiidina (as in Fig. 7.2) comes from combined morphological and nucleotide sequence data from several genes. Phasmatodea conventionally have been classified in three families (although some workers raise many subfamilies to family rank). The only certainty in internal relationships is that plesiomorphic western North American Timema is sister to the remaining extant members of the order (termed Euphasmida). An interpretation of recent nucleotide sequence data suggests that Phasmatodea ancestrally were wingless and flightedness may have re-evolved several to many times in the radiation of the order.
Order Embiidina (= Embioptera) (embiids, webspinners) (see also Box 9.5)
Embiidina have an elongate, cylindrical body, somewhat flattened in the male. The head has kidney-shaped compound eyes that are larger in males than females, and lacks ocelli. The antennae are multi- segmented and the mandibulate mouthparts project forwards (prognathy). All females and some males are apterous; but if present, the wings are characteristically soft and flexible, with blood sinus veins stiffened for flight by blood pressure. The legs are short, with three-segmented tarsi, and the basal segment of each fore tarsus is swollen because it contains silk glands. The hind femora are swollen by strong tibial muscles. The abdomen is 10-segmented with rudiments of segment 11 and with two-segmented cerci. The female external genitalia are simple (no ovipositor), and those of males are complex and asymmetrical.
Embiids are undoubtedly monophyletic based above all on the ability to produce silk from unicellular glands in the anterior basal tarsus. A general morphological resemblance to Plecoptera based on reduced phallomeres, a trochantin-episternal sulcus, and separate coxopleuron and premental lobes is not supported by nucleotide sequences that instead imply a sister-group relationship with Phasmatodea. Internal relationships amongst the described higher taxa of Embiidina suggest that the prevailing classification into eight families includes many non-monophyletic groups. Evidently, much further study is needed to understand relation- ships within Embiidina, and among it and other neopterans.
Order Dermaptera (earwigs) (see also Box 9.7)
Adult earwigs are elongate and dorsoventrally flattened with mandibulate, forward-projecting mouthparts, compound eyes ranging from large to absent, no ocelli, and short annulate antennae. The tarsi are three-segmented with a short second tarsomere. Many species are apterous or, if winged, the fore wings are small, leathery, and smooth, forming unveined tegmina, and the hind wings are large, membranous, semi-circular, and dominated by an anal fan of radiating vein branches connected by cross-veins.
The five species commensal or ectoparasitic on bats in south-east Asia were placed in suborder Arixeniina. A few species semi-parasitic on African rodents were placed in suborder Hemimerina. Earwigs in both of these groups are blind, apterous, and exhibit pseudo- placental viviparity. Recent morphological study of Hemimerina suggests derivation from within Forficulina, rendering that suborder paraphyletic. The relationships of Arixeniina to more “typical” earwigs (Forficulina) are uninvestigated. Within Forficulina, only four (Karshiellidae, Apachyidae, Chelisochidae, and Forficulidae) of eight or nine families proposed appear to be supported by synapomorphies. Other families may not be monophyletic, as much weight has been placed on plesiomorphies, especially of the penis specifically and genitalia more generally, or homoplasies (convergences) in furcula form and wing reduction.
A sister-group relationship to Dictyoptera that is well supported on morphology, including many features of the wing venation, is not supported by nucleotide sequences that demonstrate an earlier-branching sister- group relationship to Zoraptera (Fig. 7.2). Whether the pair of orders is considered part of Polyneoptera or sister to the remainder of Neoptera is as yet unclear, and the relationship is best shown as unresolved.
Order Zoraptera (zorapterans) (see also Box 9.6) Zoraptera is one of the smallest and probably the least known pterygote order. Zorapterans are small, rather termite-like insects, with simple morphology. They have biting, generalized mouthparts, including five-segmented maxillary palps and three-segmented labial palps. Sometimes both sexes are apterous, and in alate forms the hind wings are smaller than the fore wings; the wings are shed as in ants and termites. Wing venation is highly specialized and reduced.
Traditionally the order contained only one family (Zorotypidae) and one genus (Zorotypus), but has been divided into several genera of uncertain monophyly, delimited predominantly on wing venation. The phylogenetic position of Zoraptera based on morphology has been controversial, ranging through membership of the hemipteroid orders, sister to Isoptera, an orthopteroid, or a blattoid. Wing shape and venation resembles that of narrow-winged Isoptera, and analysis of major wing structures and musculature imply Zoraptera belong in a wide “blattoid” lineage. Hind-leg musculature revealed a derived condition shared only by Embiidina. Cephalic, abdominal, and nucleotide char- acters indicate an early divergence, perhaps as sister to Dermaptera, originating before the origin of the Dictyoptera clade.
Subdivision Paraneoptera (Acercaria, or Hemipteroid assemblage)
This subdivision comprises the orders Psocoptera, Phthiraptera, Thysanoptera, and Hemiptera. This group is defined by derived features of the mouthparts, including the slender, elongate maxillary lacinia separated from the stipes and a swollen postclypeus containing an enlarged cibarium (sucking pump), and the reduction in tarsomere number to three or less.
Within Paraneoptera, the monophyletic superorder Psocodea contains Phthiraptera (parasitic lice) and Psocoptera (booklice). Phthiraptera is monophyletic, but the clade arose from within Psocoptera, rendering that group paraphyletic. Although sperm morphology and some molecular sequence data imply that Hemiptera is sister to Psocodea + Thysanoptera, a grouping of Thysanoptera + Hemiptera (called superorder Condylognatha) is supported by derived head and mouthparts including the stylet mouthparts, features of the wing base, and the presence of a sclerotized ring between antennal flagellomeres. Condylognatha thus forms the sister group to Psocodea.
Order Psocoptera (psocids, barklice, booklice) (see also Box 11.9)
Psocoptera is a worldwide order of cryptic small insects, with a large, mobile head, bulbous postclypeus, and membranous wings held roof-like over the abdomen. Evidently, Psocoptera belong with Phthiraptera in a monophyletic clade Psocodea. However, Psocoptera is rendered paraphyletic by a postulated relationship of Phthiraptera to the psocopteran family Liposcelidae. Internal relationships of the more than 30 families of psocids are poorly known and of the three suborders, Troctomorpha, Trogiomorpha, and Psocomorpha, there is support only for the monophyly of Psocomorpha.
Order Phthiraptera (parasitic lice) (see also Box 15.3) Phthirapterans are wingless obligate ectoparasites of birds and mammals. Monophyly of, and relationships among, traditional suborders Anoplura, Amblycera, Ischnocera, and Rhyncophthirina are poorly understood and nearly all possible arrangements have been proposed. The latter three suborders have been treated as a monophyletic Mallophaga (biting and chewing lice) based on their feeding mode and morphology, in contrast to the piercing and blood-feeding Anoplura. Cladistic analysis of morphology has disputed mal- lophagan monophyly, suggesting the relationship Amblycera (Ischnocera (Anoplura + Rhyncophthirina)). Ignorance of robust estimates of relationship restricts estimation of evolutionary interactions, such as co-spe- ciation, between lice and their bird and mammal hosts.
Order Thysanoptera (thrips) (see also Box 11.7)
The development of Thysanoptera is intermediate between hemi- and holometabolous. Their head is elongate and the mouthparts are unique in that the maxillary laciniae form grooved stylets, the right mandible is atrophied, but the left mandible forms a stylet; all three stylets together form the feeding apparatus. The tarsi are one- or two-segmented, and the pretarsus has an apical protrusible adhesive ariolum (bladder or vesicle). Reproduction in thrips is haplodiploid.
Limited molecular evidence supports a traditional morphological division of the Thysanoptera into two suborders, Tubulifera containing a sole, speciose, family Phlaeothripidae, and Terebrantia. Terebrantia includes one speciose family, Thripidae, and seven smaller families. Relationships among families in Terebrantia are poorly resolved, although phylogenies are being generated at lower levels concerning aspects of the evolution of sociality, especially the origins of gall-inducing thrips, and of “soldier” castes in Australian gall-inducing Thripidae.
Order Hemiptera (bugs, cicadas, leafhoppers, planthoppers, spittle bugs, aphids, jumping plant lice, scale insects, whiteflies, moss bugs) (see also Boxes 10.6 & 11.8) Hemiptera, the largest non-endopterygote order, has diagnostic mouthparts, with mandibles and maxillae modified as needle-like stylets, lying in a beak-like, grooved labium, collectively forming a rostrum or proboscis. Within this, the stylet bundle contains two canals, one delivering saliva and the other uptaking fluid. Hemiptera lack maxillary and labial palps. The prothorax and mesothorax usually are large and the metathorax small. Venation of both pairs of wings can be reduced; some species are apterous, and male scale insects have only one pair of wings. Legs often have complex pretarsal adhesive structures. Cerci are lacking.
Hemiptera and Thysanoptera are sister groups within Paraneoptera. Hemiptera once was divided into two groups, Heteroptera (true bugs) and “Homoptera” (cicadas, leafhoppers, planthoppers, spittle bugs, aphids, psylloids, scale insects, and whiteflies), treated as either suborders or as orders. All “homopterans” are terrestrial plant feeders and many share a common biology of producing honeydew and being ant- attended. Although sharing defining features, such as wings held roof-like over the abdomen, fore wings either membranous or in the form of tegmina of uni- form texture, and with the rostrum arising ventrally close to the anterior of the thorax, “Homoptera” repres- ents a paraphyletic grade rather than a clade (Fig. 7.5). This view finds support in reinterpreted morphological data and from analyses of nucleotide sequences, which also suggest more complicated relationships among the higher groups of hemipterans (Fig. 7.5).
The rank of hemipteran clades has been much dis- puted. We follow a system of five suborders recognized on phylogenetic grounds. Fulgoromorpha, Cicadomorpha, Coleorrhyncha, and Heteroptera (collectively termed the Euhemiptera) form the sister group to suborder Sternorrhyncha. The latter contains the aphids (Aphidoidea), jumping plant lice (Psylloidea), scale insects (Coccoidea), and whiteflies (Aleyrodoidea), which are characterized principally by their possession of a particular kind of gut filter chamber, a rostrum that appears to arise between the bases of their front legs and, if winged, by absence of the vannus and vannal fold in the hind wings. Some relationships among Euhemiptera are unsettled. A traditional grouping called the Auchenorrhyncha, morphologically defined by their possession of a tymbal acoustic system, an aristate antennal flagellum, and reduction of the prox- imal median plate in the wing base, contains the Fulgoromorpha (planthoppers) and Cicadomorpha (cicadas, leafhoppers, and spittle bugs). Paleontological data combined with nucleotide sequences suggest that Cicadomorpha is sister to Coleorrhyncha + Heteroptera (sometimes called Prosorrhyncha), which would ren- der Auchenorrhyncha paraphyletic. However, relationships among Cicadomorpha, Fulgoromorpha, and Coleorrhyncha + Heteroptera are still disputed and thus are portrayed here as an unresolved trichotomy (Fig. 7.5).
Heteroptera (true bugs, including assassin bugs, back-swimmers, lace bugs, stink bugs, waterstriders, and others) has as its sister group the Coleorrhyncha, containing only one family, Peloridiidae or moss bugs. Although small, cryptic and rarely collected, moss bugs have generated considerable phylogenetic interest due to their combination of ancestral and derived hemipteran features, and their exclusively “relictual” Gondwanan distribution. Heteropteran diversity is distributed amongst about 80 families, forming the largest hemipteran clade. Heteroptera is diagnosed most easily by the presence of metapleural scent glands, and monophyly is undisputed.
Subdivision Endopterygota (= Holometabola)
Endopterygota comprise insects with holometabolous development in which immature (larval) instars are very different from their respective adults. The adult wings and genitalia are internalized in their pre-adult expression, developing in imaginal discs that are evaginated at the penultimate molt. Larvae lack true ocelli. The “resting stage” or pupa is non-feeding, and precedes an often active pharate (“cloaked” in pupal cuticle) adult. Unique derived features are less evident in adults than in earlier developmental stages, but the clade is recovered consistently from all phylogenetic analyses.
Two or three groups currently are proposed amongst the endopterygotes, of which one of the strongest is a sister-group relationship termed Amphiesmenoptera between the Trichoptera (caddisflies) and Lepidoptera (butterflies and moths). A plausible scenario of an ancestral amphiesmenopteran taxon envisages a larva living in damp soil amongst liverworts and mosses followed by radiation into water (Trichoptera) or into terrestrial plant-feeding (Lepidoptera).
A second strongly supported relationship is between three orders: Neuroptera, Megaloptera, and Raphidioptera, called Neuropterida and sometimes treated as a group of ordinal rank, which shows a sister-group relationship to Coleoptera.
A third, postulated relationship — Antliophora — unites Diptera (true flies), Siphonaptera (fleas), and Mecoptera (scorpionflies and hangingflies). Their relationships, particularly concerning Siphonaptera, have been debated. Fleas were considered as sister group to Diptera, but anatomical and nucleotide sequence evidence increasingly points to a relationship with the curious-looking mecopterans, the snow fleas of the family Boreidae (Fig. 7.6).
Strepsiptera is phylogenetically enigmatic, but resemblance of their first-instar larvae (called triun-gulins) to those of certain Coleoptera, notably parasitic Rhipiphoridae, and some wing-base features have been cited as indicative of a close relationship. This suggested placement is becoming less likely, as molecular evidence (and haltere development) suggests alternatives, either with Diptera or distant from either Diptera or Coleoptera. Strepsiptera has undergone much morphological and molecular evolution, and is highly derived with few features shared with any other taxon. Such long-isolated evolution of the genome can create a problem known as “long-branch attraction”, in which nucleotide sequences may converge by chance mutations alone with those of an unrelated taxon with a similarly long independent evolution, for the strep-sipteran notably with Diptera. The issue of relationship remains unresolved, although morphological study of wing-base morphology suggests that proximity to neither Diptera nor Coleoptera is likely.
The relationships of two major orders of endopterygotes, Coleoptera and Hymenoptera, remain to be considered. Several positions have been proposed for Coleoptera but current evidence derived from female genitalia and ambivalent evidence from eye structure supports a sister-group relationship to Neuropterida. This group is sister to the remaining Endopterygota in many analyses. Hymenoptera may be the sister to Antliophora + Amphiesmenoptera, but the many highly derived features of adults and reductions in larvae limit morphological justification for this position.
Within the limits of uncertainty, the relationships within Endopterygota are summarized in Fig. 7.2, in which uncertain or ambiguous associations are shown by interrupted lines and suspect paraphyletic taxon names are italicized.
Order Coleoptera (beetles) (see also Boxes 10.6 & 11.10) Coleoptera undoubtedly lie amongst early branches of the Endopterygota. The major shared derived feature of Coleoptera is the development of the fore wings as sclerotized rigid elytra, which extend to cover some or many of the abdominal segments, and beneath which the propulsive hind wings are elaborately folded when at rest. Some molecular studies show Coleoptera polyphyletic or paraphyletic with respect to some or all of Neuropterida. However, this is impossible to reconcile with the morphological support for coleopteran monophyly, and we accept that a sister-group relationship to Neuropterida is most probable.
Within Coleoptera, four modern lineages (treated as suborders) are recognized: Archostemata, Adephaga, Polyphaga, and Myxophaga. Archostemata includes only the small families Ommatidae, Crowsoniellidae, Cupedidae, and Micromalthidae, and probably forms the sister group to the remaining extant Coleoptera. The few known larvae are wood-miners with a sclerotized ligula and a large mola on each mandible. Adults have movable hind coxae with usually visible trochantins, and five (not six) ventral abdominal plates (ventrites), but share with Myxophaga and Adephaga wing folding features, lack of any cervical sclerites, and an external prothoracic pleuron. In contrast to Myxophaga, the pretarsus and tarsus are unfused.
Adephaga is diverse, second in size only to Polyphaga, and includes ground beetles, tiger beetles, whirligigs, predaceous diving beetles, and wrinkled bark beetles, amongst others. Larval mouthparts are adapted for liquid-feeding, with a fused labrum and no mandibular mola. Adults have the notopleural sutures visible on the prothorax and have six visible abdominal sterna with the first three fused into a single ventrite which is divided by the hind coxae. Pygidial defense glands are widespread in adults. The most speciose included family is Carabidae, or ground beetles, with a predominantly predaceous feeding habit, but Adephaga also includes the aquatic families, Dytiscidae, Gyrinidae, Haliplidae and Noteridae, and the mycophagous Rhysodidae, or wrinkled bark beetles. Morphology suggests that Adephaga is sister group to the combined Myxophaga and Polyphaga, although some nucleotide sequences suggest Adephaga as sister to Polyphaga, with Myxophaga sister to the two combined.
Myxophaga is a clade of small, primarily riparian aquatic beetles, comprising families Lepiceridae, Torridincolidae, Hydroscaphidae, and Microsporidae, united by the synapomorphic fusion of the pretarsus and tarsus. The three-segmented larval antenna, five-segmented larval legs with a single pretarsal claw, fusion of trochantin with the pleuron, and ventrite structure support a sister-group relationship of Myxophaga with the Polyphaga. This has been challenged by some workers, notably because some interpreta- tions of wing venation and folding support Polyphaga (Archostemata (Myxophaga + Adephaga)).
Polyphaga contains the majority (>90% of species) of beetle diversity, with about 300,000 described species. The suborder includes rove beetles (Staphylinoidea), scarabs and stag beetles (Scarabaeoidea), metallic wood-boring beetles (Buprestoidea), click beetles and fireflies (Elateroidea), as well as the diverse Cucujiformia, including fungus beetles, grain beetles, ladybird beetles, darkling beetles, blister beetles, longhorn beetles, leaf beetles, and weevils. The prothoracic pleuron is not visible externally, but is fused with the trochantin and remnant internally as a “cryptopleuron”. Thus, one suture between the notum and the sternum is visible in the prothorax in polyphagans, whereas two sutures (the sternopleural and notopleural) often are visible externally in other suborders (unless secondary fusion between the sclerites obscures the sutures, as in Micromalthus). The transverse fold of the hind wing never crosses the media posterior (MP) vein, cervical sclerites are present, and hind coxae are mobile and do not divide the first ventrite. Female polyphagan beetles have telotrophic ovarioles, which is a derived condition within beetles.
The internal classification of Polyphaga involves several superfamilies or series, whose constituents are relatively stable, although some smaller families (whose rank even is disputed) are allocated to different clades by different authors. Large superfamilies include Hydrophiloidea, Staphylinoidea, Scarabaeoidea, Buprestoidea, Byrrhoidea, Elateroidea, Bostrichoidea, and the grouping Cucujiformia. This latter includes the vast majority of phytophagous (plant-eating) beetles, united by cryptonephric Malpighian tubules of the normal type, the eye with a cone ommatidium with open rhabdom, and lack of functional spiracles on the eighth abdominal segment. Constituent superfamilies of Cucujiformia are Cleroidea, Cucujoidea, Tenebrionoidea, Chrysomeloidea, and Curculionoidea. Evidently, adoption of a phytophagous lifestyle correlates with speciosity in beetles, with Cucujiformia, especially weevils (Curculionoidea), forming a major radiation (see section 8.6).
Neuropterida, or neuropteroid orders
Orders Megaloptera (alderflies, dobsonflies, fishflies), Raphidioptera (snakeflies), and Neuroptera (lacewings, antlions, owlflies) (see also Boxes 10.6 & 13.4) Neuropterida comprise three minor (species-poor) orders, whose adults have multisegmented antennae, large, separated eyes, and mandibulate mouthparts. The prothorax may be larger than either the meso- or metathorax, which are about equal in size. Legs some- times are modified for predation. The fore and hind wings are quite similar in shape and venation, with folded wings often extending beyond the abdomen. The abdomen lacks cerci.
Megalopterans are predatory only in the aquatic larval stage; although adults have strong mandibles, they are not used in feeding. Adults closely resemble neuropterans, except for the presence of an anal fold in the hind wing. Raphidiopterans are terrestrial predators as adults and larvae. The adult is mantid-like, with an elongate prothorax, and the head is mobile and used to strike, snake-like, at prey. The larval head is large and forwardly directed. Many adult neuropterans are predators, and have wings typically characterized by numerous cross-veins and “twigging” at the ends of veins. Neuropteran larvae usually are active predators with slender, elongate mandibles and maxillae com- bined to form piercing and sucking mouthparts.
Megaloptera, Raphidioptera, and Neuroptera may be treated as separate orders, united in Neuropterida, or Raphidioptera may be included in Megaloptera. Neuropterida undoubtedly is monophyletic with new support from morphology of the wing-base sclerites. This latter feature also supports the long-held view that Neuropterida forms a sister group to Coleoptera. Each component appears monophyletic, although a doubt remains concerning megalopteran monophyly. There remains uncertainty about internal relationships, which traditionally have Megaloptera and Raphidioptera as sister groups. Recent reanalyses with some new character suites propose Megaloptera as sister to Neuroptera with a novel scenario of ancestral aquatic larvae (as seen in Sisyridae within Neuroptera, and in all Megaloptera) in Neuropterida.
Order Strepsiptera (see also Box 13.6)
Strepsiptera form an enigmatic order showing extreme sexual dimorphism. The male’s head has bulging eyes comprising few large facets and lacks ocelli; the antennae are flabellate or branched, with four to seven segments. The fore wings are stubby and lack veins, whereas the hind wings are broadly fan-shaped, with few radiating veins; the legs lack trochanters and often also claws. Females are either coccoid-like or larviform, wingless, and usually retained in a pharate (cloaked) state, protruding from the host. The first-instar larva is a triungulin, without antennae and mandibles, but with three pairs of thoracic legs; subsequent instars are maggot-like, lacking mouthparts or appendages. The pupa, which has immovable mandibles but appendages free from its body, develops within a puparium formed from the last larval instar.
The phylogenetic position of Strepsiptera has been subject to much speculation because modifications associated with their endoparasitic lifestyle mean that few characteristics are shared with possible relatives. In having posteromotor flight (using only metathoracic wings) they resemble Coleoptera, but other putative synapomorphies with Coleoptera appear suspect or mistaken. The fore-wing-derived halteres of strep- sipterans are gyroscopic organs of equilibrium with the same functional role as the halteres of Diptera (although the latter are derived from the hind wing). Nucleotide sequence studies indicate that Strepsiptera might be a sister group to Diptera, which is one relationship indicated on Fig. 7.2 by the broken line.
Order Mecoptera (scorpionflies, hangingflies) (see also Box 13.5)
Mecopteran adults have an elongate, ventrally projecting rostrum, containing elongate, slender mandibles and maxillae, and an elongate labium. The eyes are large and separated, the antennae filiform and multi-segmented. The fore and hind wings are narrow, similar in size, shape, and venation, but often are reduced or absent. The legs may be modified for predation. Larvae have a heavily sclerotized head capsule, are mandibulate, and may have compound eyes comprising three to 30 ocelli (absent in Panorpidae, indistinct in Nannochoristidae). The thoracic segments are about equal, and have short thoracic legs with fused tibia and tarsus and a single claw. Prolegs usually are present on abdominal segments 1–8, and the terminal segment (10) has either paired hooks or a suction disk. The pupa is immobile, mandibulate, and with appendages free.
Although some adult Mecoptera resemble neuropterans, strong evidence supports a relationship to Diptera. Intriguing recent morphological studies, plus robust evidence from molecular sequences, suggest that Siphonaptera arose from within Mecoptera, as a sister group to the “snow fleas” (Boreidae) (Fig. 7.6). The phylogenetic position of Nannochoristidae, a southern hemisphere mecopteran taxon currently treated as being of subfamily rank, has a significant bearing on internal relationships within Antliophora. Nucleotide sequence data suggest that it is sister to Boreidae + Siphonaptera, and therefore is of equivalent rank to the boreids, fleas, and the residue of Mecoptera (sensu stricto) — and logically each should be treated as orders, or Siphonaptera reduced in rank within Mecoptera.
Order Siphonaptera (fleas) (see also Box 15.4) Siphonaptera are bilaterally compressed, apterous ectoparasites, with mouthparts specialized for piercing and sucking, lacking mandibles but with an unpaired labral stylet and two elongate serrate, lacinial stylets that together lie within a maxillary sheath. A salivary pump injects saliva into the wound, and cibarial and pharyngeal pumps suck up the blood meal. Fleas lack compound eyes and the antennae lie in deep lateral grooves. The body is armed with many posteriorly directed setae and spines, some of which form combs, especially on the head and anterior thorax. The meta- thorax houses very large muscles associated with the long and strong hind legs, which power the prodigious leaps made by these insects.
After early suggestions that the fleas arose from a mecopteran, the weight of evidence suggested they formed the sister group to Diptera. However, increasing molecular and novel morphological evidence now points to a sister-group relationship to only part of Mecoptera, specifically the Boreidae (snow fleas) (Fig. 7.6). Internal relationships of the fleas are under study and preliminary results imply that monophyly of many families is uncertain.
Order Diptera (true flies) (see also Boxes 5.4, 10.5, & 15.5)
Diptera are readily recognized by the development of hind (metathoracic) wings as balancers, or halteres (halters), and in the larval stages by a lack of true legs and the often maggot-like appearance. Venation of the fore (mesothoracic), flying wings ranges from complex to extremely simple. Mouthparts range from biting-and-sucking (e.g. biting midges and mosquitoes) to “lapping”-type with a pair of pseudotracheate labella functioning as a sponge (e.g. house flies). Dipteran larvae lack true legs, although various kinds of locomotory apparatus range from unsegmented pseudolegs to creeping welts on maggots. The larval head capsule may be complete, partially undeveloped, or completely absent in a maggot head that consists only of the internal sclerotized mandibles (“mouth hooks”) and supporting structures.
Traditionally, Diptera had two suborders, Nematocera (crane flies, midges, mosquitoes, and gnats) with a slender, multisegmented antennal flagellum, and heavier-built Brachycera (“higher flies” including hover flies, blow flies, and dung flies) with a shorter, stouter, and fewer-segmented antenna. However, Brachycera is sister to only part of “Nematocera”, and thus Nematocera is paraphyletic.
Internal relationships amongst Diptera are becoming better understood, although with some notable exceptions. Ideas concerning early branches in dipteran phylogeny are inconsistent. Traditionally, Tipulidae (or Tipulomorpha) is a first-branching clade on evidence from the wing and other morphology. Such an arrangement is difficult to reconcile with the much more derived larva, in which the head capsule is variably reduced. Furthermore, some molecular evidence casts doubt on this position for the crane flies, but as yet does not produce a robust estimate for any alternative early-branching pattern. Alternative views based on morphology have suggested that the relictual family Tanyderidae, with complex (“primitive”) wing venation, arose early in the diversification of the order. Support comes also from the tanyderid larval morphology, and putative placement in Psychodomorpha, considered a probable early-branching clade.
There is strong support for a grouping called Culicomorpha, comprising mosquitoes (Culicidae) and their relatives (Corethrellidae, Chaoboridae, Dixidae) and their sister group the black flies, midges, and relatives (Simuliidae, Thaumaleidae, Ceratopogonidae, Chironomidae), and for Bibionomorpha, comprising the fungus gnats (Mycetophilidae, Bibionidae, Anisopodidae, and possibly Cecidomyiidae (gall midges)).
Monophyly of Brachycera, comprising “higher flies”, is established by features including the larva having a posterior elongate head contained within the protho- rax, a divided mandible and loss of premandible, and in the adult by eight or fewer antennal flagellomeres, two or fewer palp segments, and separation of the male gen- italia into two parts (epandrium and hypandrium). All relationships of Brachycera are to a subgroup within “Nematocera”, perhaps as sister to Psychodomorpha or even to Culicomorpha (molecular data only), but strong support is provided for a sister relationship to the Bibionomorpha, or to a group within the Anisopodidae. Brachycera contains four equivalent groups with internally unresolved relationships: Tabanomorpha (with a brush on the larval mandible and the larval head retractile); Stratiomyomorpha (with larval cuticle calcified and pupation in last-larval instar exuviae); Xylophagomorpha (with a distinctive elongate, conical, strongly sclerotized larval head capsule, and abdomen posteriorly ending in a sclerotized plate with terminal hooks); and Muscomorpha (adults with tibial spurs absent, flagellum with no more than four flagellomeres, and female cercus single-segmented). This latter speciose group contains Asiloidea (robber flies, bee flies, and relatives) and Eremoneura (Empidoidea and Cyclorrhapha). Eremoneura is a strongly supported clade based on wing venation (loss or fusion of vein M4 and closure of anal cell before margin), presence of ocellar setae, unitary palp and genitalic features, plus larval stage with only three instars and maxillary reduction. Cyclorrhaphans, united by metamorphosis in a puparium formed by the last instar larval skin, include a heterogeneous group including Syrphidae (hover flies) and the Schizophora defined by the presence of a balloon-like ptilinum that everts from the frons to assist the adult escape the puparium. Within Schizophora, the “higher” cyclorrhaphans include the ecologically very diverse acalypterates, and the blow flies and relatives (Calypteratae).
Order Hymenoptera (ants, bees, wasps, sawflies, and wood wasps) (see also Box 12.2)
The mouthparts of adults are directed ventrally to forward projecting, ranging from generalized mandibulate to sucking and chewing, with mandibles often used for killing and handling prey, defense, and nest building. The compound eyes often are large; the antennae are long, multisegmented, and often prominently held forwardly or recurved dorsally. “Symphyta” (wood wasps and sawflies) has a conventional three-segmented thorax, but in Apocrita (ants, bees, and wasps) the propodeum (abdominal segment 1) is included with the thorax to form a mesosoma. The wing venation is relatively complete in large sawflies, and reduced in Apocrita in correlation with body size, such that very small species of 1–2 mm have only one divided vein, or none. In Apocrita, the second abdominal segment (and sometimes also the third) forms a constriction, or petiole (Box 12.2). Female genitalia include an ovipositor, comprising three valves and two major basal sclerites, which in aculeate Hymenoptera is modified as a sting associated with a venom apparatus.
Symphytan larvae are eruciform (caterpillar-like), with three pairs of thoracic legs bearing apical claws and with some abdominal legs. Apocritan larvae are apodous, with the head capsule frequently reduced but with prominent strong mandibles.
Hymenoptera forms the sister group to Amphies- menoptera (= Trichoptera + Lepidoptera) + Antliophora (= Diptera + Mecoptera/Siphonaptera) (Fig. 7.2), although an earlier-branching position in the Holometabola has been advocated. Hymenoptera often are treated as containing two suborders, Symphyta (wood wasps and sawflies) and Apocrita (wasps, bees, and ants). However, Apocrita appears to be sister to one family of symphytan only, the Orussidae, and thus “symphytans” form a paraphyletic group.
Within Apocrita, aculeate (Aculeata) and parasitic (Parasitica or terebrant) wasp groups were considered each to be monophyletic, but aculeates evidently origin- ated from within a paraphyletic Parasitica. Internal relationships of aculeates, including vespids (paper wasps, yellow jackets, etc.), formicids (ants), and apids (bees), and the monophyly of subordinate groups are under scrutiny. Apidae evidently arose as sister to, or from within, Sphecidae (digger wasps), but the precise relationships of another significant group of aculeates, Formicidae (ants), within Vespoidea are less certain (Fig. 12.2).
Order Trichoptera (caddisflies) (see also Box 10.4)
The moth-like adult trichopteran has reduced mouthparts lacking any proboscis, but with three- to five-segmented maxillary palps and three-segmented labial palps. The antennae are multisegmented and filiform and often as long as the wings. The compound eyes are large, and there are two to three ocelli. The wings are haired or less often scaled, and differentiated from all but the most basal Lepidoptera by the looped anal veins in the fore wing, and absence of a discal cell. The larva is aquatic, has fully developed mouthparts, three pairs of thoracic legs (each with at least five segments), and lacks the ventral prolegs characteristic of lepidopteran larvae. The abdomen terminates in hook-bearing prolegs. The tracheal system is closed, and associated with tracheal gills on most abdominal segments. The pupa also is aquatic, enclosed in a retreat often made of silk, with functional mandibles that aid in emergence from the sealed case.
Amphiesmenoptera (Trichoptera + Lepidoptera) is now unchallenged, despite earlier suggestions that Trichoptera may have originated within Lepidoptera. Proposed internal relationships within the Trichoptera range from stable and well supported, to unstable and anecdotal. Monophyly of suborder Annulipalpia (comprising families Hydropsychidae, Polycentropodidae, Philopotamidae, and some close relatives) is well supported by larval and adult morphology — including presence of an annulate apical segment of both adult maxillary and larval palp, absence of male phallic parameres, presence of papillae lateral to the female cerci, and in the larva by the presence of elongate anal hooks and reduced abdominal tergite 10.
The monophyly of the case-making suborder Integripalpia (comprising families Phryganeidae, Limnephilidae, Leptoceridae, Sericostomatidae, and relatives) is supported by the absence of the m cross-vein, hind wings broader than fore wings especially in the anal area, female lacking both segment 11 and cerci, and larval character states including usually complete sclerotization of the mesonotum, hind legs with lateral projection, lateral and mid-dorsal humps on abdominal segment 1, and short and stout anal hooks.
Monophyly of a third putative suborder, Spicipalpia, is more contentious. Defined for a grouping of families Glossosomatidae, Hydroptilidae, and Rhyacophilidae (and perhaps the Hydrobiosidae), uniting features are the spiculate apex of the adult maxillary and labial palps, the ovoid second segment of the maxillary palp, and an eversible oviscapt (egg-laying appendage). Morphological and molecular evidence fail to confirm Spicipalpia monophyly, unless at least Hydroptilidae is removed.
All possible relationships between Annulipalpia, Integripalpia, and Spicipalpia have been proposed, sometimes associated with scenarios concerning the evolution of case-making. An early idea that Annulipalpia are sister to a paraphyletic Spicipalpia + mono- phyletic Integripalpia finds support from some morphological and molecular data.
Order Lepidoptera (moths and butterflies) (see also Box 11.11)
Adult heads bear a long, coiled proboscis formed from greatly elongated maxillary galeae; large labial palps usually are present, but other mouthparts are absent, except that mandibles are present primitively in some groups. The compound eyes are large, and ocelli usually are present. The multisegmented antennae often are pectinate in moths and knobbed or clubbed in butterflies. The wings are covered completely with a double layer of scales (flattened modified macrotrichia), and the hind and fore wings are linked by either a frenulum, a jugum, or simple overlap. Lepidopteran larvae have a sclerotized head capsule with mandib- ulate mouthparts, usually six lateral ocelli, and short three-segmented antennae. The thoracic legs are five-segmented with single claws, and the abdomen has 10 segments with short prolegs on some segments. Silk gland products are extruded from a characteristic spinneret at the median apex of the labial prementum. The pupa usually is contained within a silken cocoon.
The early-branching events in the radiation of this large order is considered well-enough resolved to serve as a test for the ability of particular nucleotide sequences to recover the expected phylogeny. Although more than 98% of the species of Lepidoptera belong in Ditrysia, the morphological diversity is concentrated in a small non-ditrysian grade. Three of the four suborders are species-poor early branches, each with just a single family (Micropterigidae, Agathiphagidae, Heterobathmiidae); these lack the synapomorphy of the mega-diverse fourth suborder Glossata, namely the characteristically developed coiled proboscis formed from the fused galea (Fig. 2.12). The highly speciose Glossata contains a comb-like branching pattern of many species-poor taxa, plus a species- rich grouping united by the larva (caterpillar) having abdominal prolegs with muscles and apical crochets (hooklets). This latter group contains the diverse Ditrysia, defined by the unique two genital openings in the female, one the ostium bursae on sternite 8, the other the genitalia proper on sternites 9 and 10. Additionally, the wing coupling is always frenulate or amplexiform and not jugate, and the wing venation tends to be heteroneuran (with venation dissimilar between fore and hind wings). Trends in the evolution of Ditrysia include elaboration of the proboscis and the reduction to loss of maxillary palpi. One of the best- supported relationships in Ditrysia is the grouping of Hesperioidea (skippers) and Papilionoidea (butterflies), united by their clubbed, dilate antennae, lack of frenulum in the wing and large humeral lobe on the hind wing. To this the neotropical Hedyloidea has been added to form the clade known as the butterflies (Fig. 7.7).

(a) in folded position, and (b) extended during prey capture with opposing hooks of the palpal lobes forming claw-like pincers. (After Wigglesworth 1964)
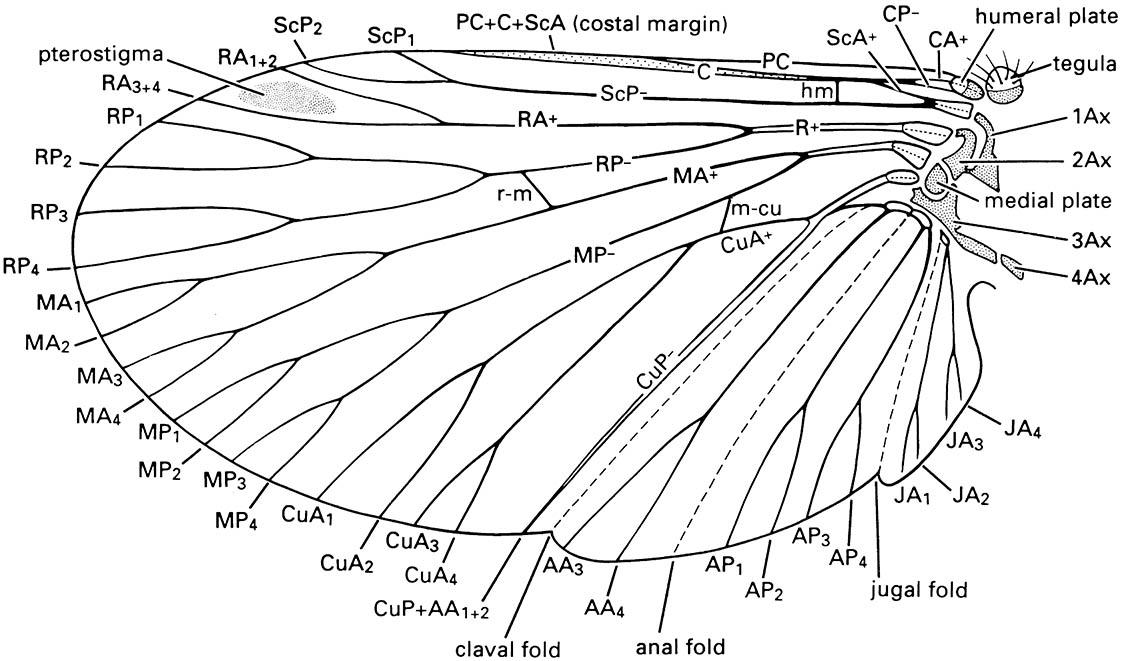
Notation as follows: AA, anal anterior; AP, anal posterior; Ax, axillary sclerite; C, costa; CA, costa anterior; CP, costa posterior; CuA, cubitus anterior; CuP, cubitus posterior; hm, humeral vein; JA, jugal anterior; MA, media anterior; m-cu, cross-vein between medial and cubital areas; MP, media posterior; PC, precosta; R, radius; RA, radius anterior; r-m, cross-vein between radial and median areas; RP, radius posterior; ScA, subcosta anterior; ScP, subcosta posterior. Branches of the anterior and posterior sector of each vein are numbered, e.g. CuA 1-4. (After CSIRO 1991)

The broken line indicates a paraphyletic taxon. (Data from several sources)
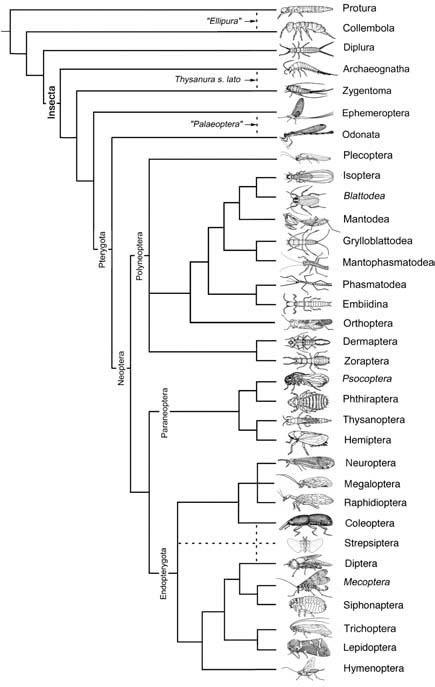
Broken lines indicate uncertain relationships. Thysanura sensu lato refers to Thysanura in the broad sense. (Data from several sources)
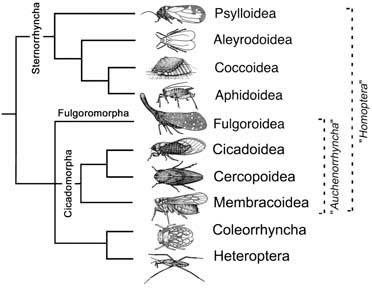
Broken lines indicate paraphyletic taxa, with names italicized. (After Bourgoin & Campbell 2002)
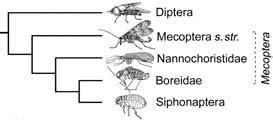
The broken lines indicate a paraphyletic taxon, with its name italicized; s. str. refers to the restricted sense. (After Whiting 2002)
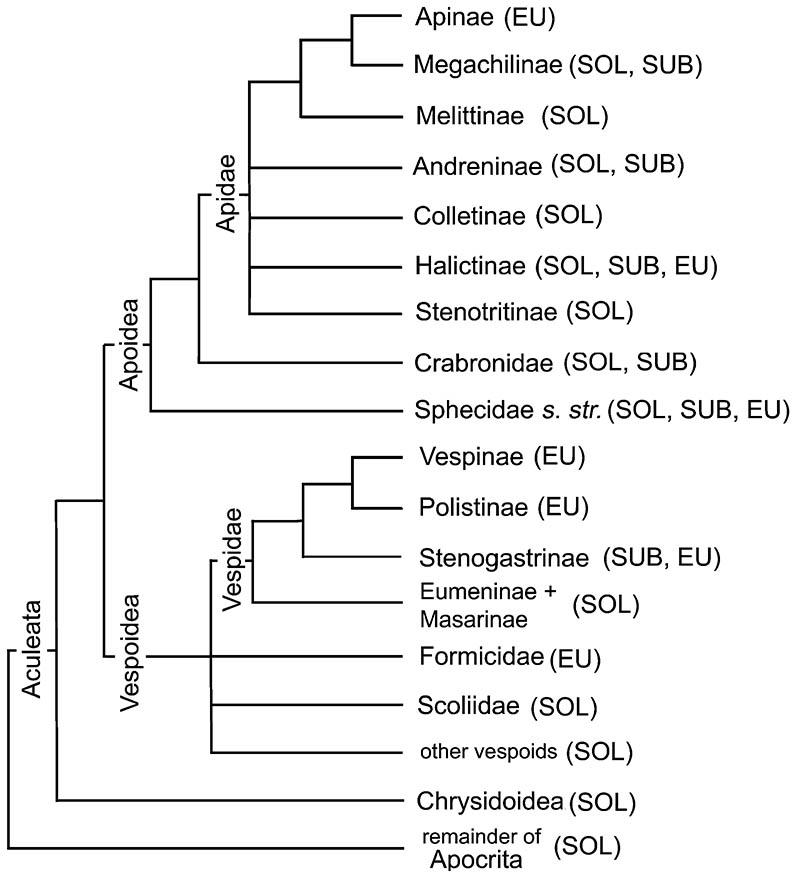
The superfamily Apoidea includes the Sphecidae sensu stricto, the Crabronidae (formerly part of a broader Sphecidae), the Ampulicidae (not shown), and all bees, here treated as one family, the Apidae, with several subfamilies (e.g. Apinae, Colletinae, Halictinae; not all solitary groups are shown) of uncertain relationships. Traditionally, bees have been classified in several families, a ranking that is unjustified phylogenetically. Probable relationships within non-social aculeate wasps (e.g. Ampulicidae, Pompilidae, and Rhopalosomatidae) and bees are not depicted. (Adapted from several sources including Gauld & Bolton 1988; Alexander 1992; Brothers 1999; B.N. Danforth, pers. comm.)
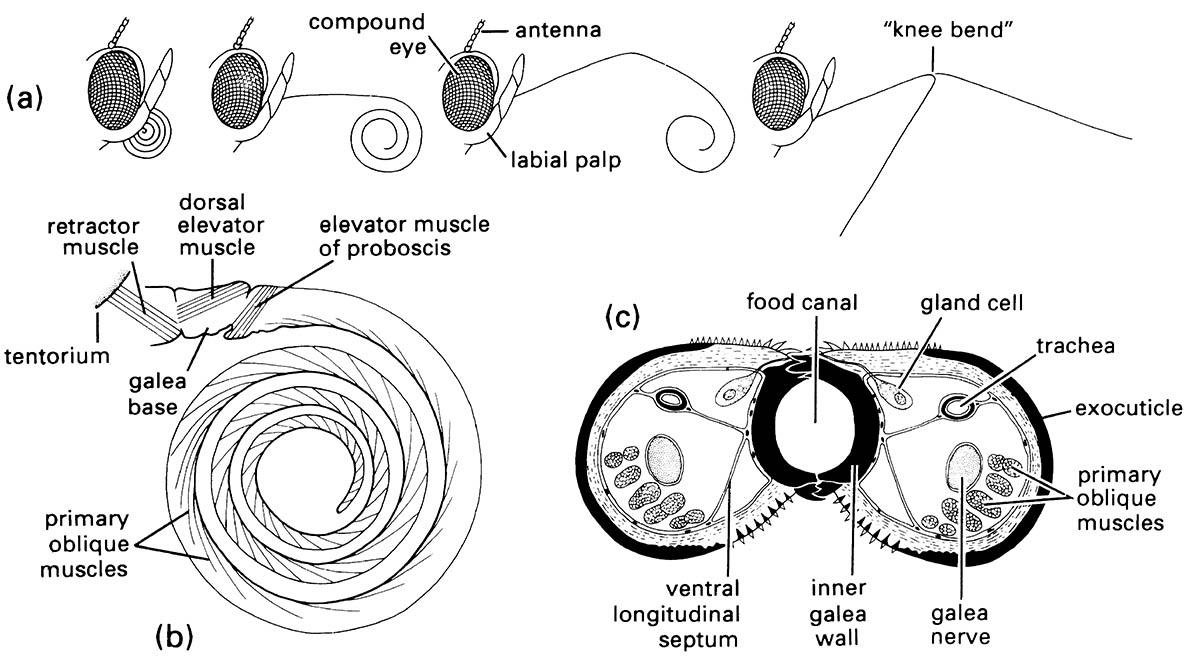
(a) Positions of the proboscis showing, from left to right, at rest, with proximal region uncoiling, with distal region uncoiling, and fully extended with tip in two of many possible different positions due to flexing at «knee bend». (b) Lateral view of proboscis musculature. (c) Transverse section of the proboscis in the proximal region. (After Eastham & Eassa 1955)
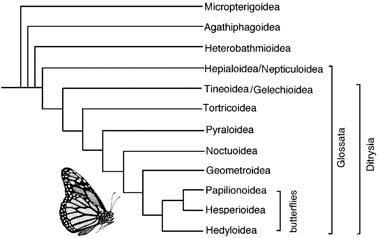
(After Kristensen & Skalski 1999)

