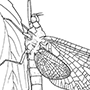
Box 10.2. Odonata (damselflies and dragonflies)
These conspicuous insects comprise a small, largely tropical order containing about 5500 described species, with about one-half belonging to the suborder Zygoptera (damselflies), and the remaining half to the suborder Anisoptera (dragonflies). Two Oriental species have been placed in a third suborder, the Anisozygoptera, which is likely invalid (section 7.4.2). The adults are medium to large (from <2 cm to >15 cm long, with a maximum wingspan of 17 cm in the South American giant damselfly (Pseudostigmatidae: Mecistogaster)). They have a mobile head with large, multifaceted compound eyes, three ocelli, short bristle-like antennae, and mandibulate mouthparts. The thorax is enlarged to accommodate the flight muscles of two pairs of elongate membranous wings that are richly veined. The slender 10-segmented abdomen terminates in clasping organs in both sexes; males possess secondary genitalia on the venter of the second to third abdominal segments; females often have an ovipositor at the ventral apex of the abdomen. In adult zygopterans the eyes are widely separated and the fore and hind wings are equal in shape with narrow bases (as illustrated in the top right figure for a lestid, Austrolestes, after Bandsma & Brandt 1963). Anisopteran adults have eyes either contiguous or slightly separated, and their wings have characteristic closed cells called the triangle (T) and hypertriangle (ht) (Fig. 2.22b); the hind wings are considerably wider at the base than the fore wings (as illustrated in the top left figure for a libellulid dragonfly, Sympetrum, after Gibbons 1986). Odonate nymphs have a variable number of up to 20 aquatic instars, with fully developed mandibulate mouthparts, including an extensible grasping labium or “mask” (Fig. 13.4). The developing wings are visible in older nymphs. The tracheal system is closed and lacks spiracles, but specialized gas-exchange surfaces are present on the abdomen as external gills (Zygoptera) or internal folds in the rectum (Anisoptera; Fig. 3.11f ). Zygopteran nymphs (such as the lestid illustrated on the lower right, after CSIRO 1970) are slender, with the head wider than the thorax, and the apex of the abdomen with three (rarely two) elongate tracheal gills (caudal lamellae). Anisopteran nymphs (such as the libellulid illustrated on the lower left, after CSIRO 1970) are more stoutly built, with the head rarely much broader than the thorax, and the abdominal apex characterized by an anal pyramid consisting of three short projections and a pair of cerci in older nymphs. Many anisopteran nymphs rapidly eject water from their anus — “jet propulsion” — as an escape mechanism.
Prior to mating, the male fills his secondary genitalia with sperm from the primary genital opening on the ninth abdominal segment. At mating, the male grasps the female by her neck or prothorax and the pair fly in tandem, usually to a perch. The female then bends her abdomen forwards to connect to the male’s secondary genitalia, thus forming the “wheel” position (as illustrated in Box 5.3). The male may displace sperm of a previous male before transferring his own (Box 5.3), and mating may last from seconds to several hours, depending on species. Egg-laying may take place with the pair still in tandem. The eggs (Fig. 5.10) are laid onto a water surface, into water, mud, or sand, or into plant tissue, depending on species. After eclosion, the hatchling (“pronymph”) immediately molts to the first true nymph, which is the first feeding stage.
The nymphs are predatory on other aquatic organisms, whereas the adults catch terrestrial aerial prey. At metamorphosis (Fig. 6.8), the pharate adult moves to the water/land surface where atmospheric gaseous exchange commences; then it crawls from the water, anchors terrestrially, and the imago emerges from the cuticle of the final-instar nymph. The imago is long-lived, active, and aerial. Nymphs occur in all waterbodies, particularly in well-oxygenated, standing waters, but elevated temperatures, organic enrichment, or in- creased sediment loads are tolerated by many species.
Phylogenetic relations are discussed in section 7.4.2 and depicted in Fig. 7.2.
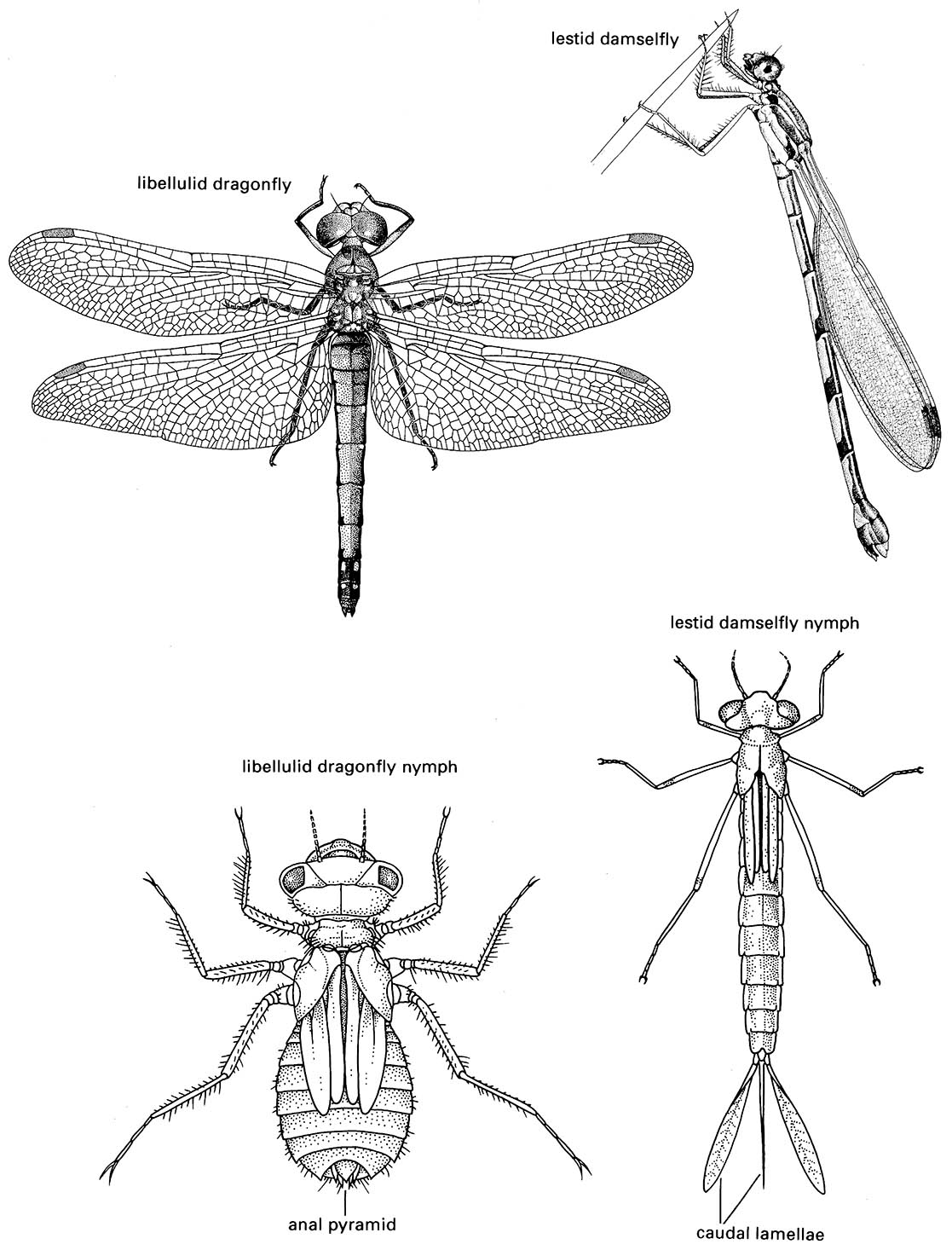
(a) fore wing of a butterfly of Danaus (Lepidoptera: Nymphalidae); (b) fore wing of a dragonfly of Urothemis (Odonata: Anisoptera: Libellulidae); (c) fore wing or tegmen of a cockroach of Periplaneta (Blattodea: Blattidae); (d) fore wing or elytron of a beetle of Anomala (Coleoptera: Scarabaeidae); (e) fore wing or hemelytron of a mirid bug (Hemiptera: Heteroptera: Miridae) showing three wing areas — the membrane, corium, and clavus; (f ) fore wing and haltere of a fly of Bibio (Diptera: Bibionidae) (after J.W.H. Trueman, unpublished. ((a-d) After Youdeowei 1977; (f) after McAlpine 1981)

(a) in folded position, and (b) extended during prey capture with opposing hooks of the palpal lobes forming claw-like pincers. (After Wigglesworth 1964)
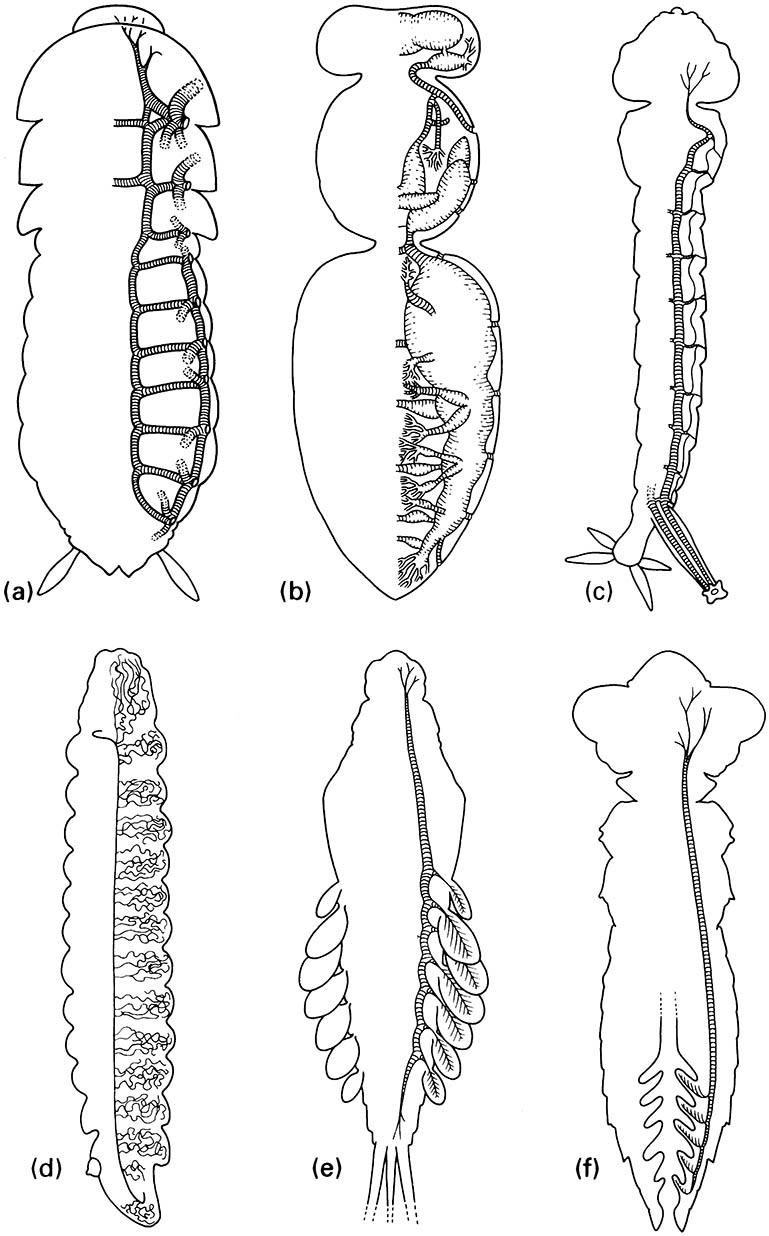
(a) Simple tracheae with valved spiracles, as in cockroaches. (b) Tracheae with mechanically ventilated air sacs, as in honey bees. (c) Metapneustic system with only terminal spiracles functional, as in mosquito larvae. (d) Entirely closed tracheal system with cutaneous gas exchange, as in most endoparasitic larvae. (e) Closed tracheal system with abdominal tracheal gills, as in mayfly nymphs. (f) Closed tracheal system with rectal tracheal gills, as in dragonfly nymphs. (After Wigglesworth 1972; details in (a) after Richards & Davies 1977, ( b) after Snodgrass 1956, (c) after Snodgrass 1935, (d) after Wigglesworth 1972)
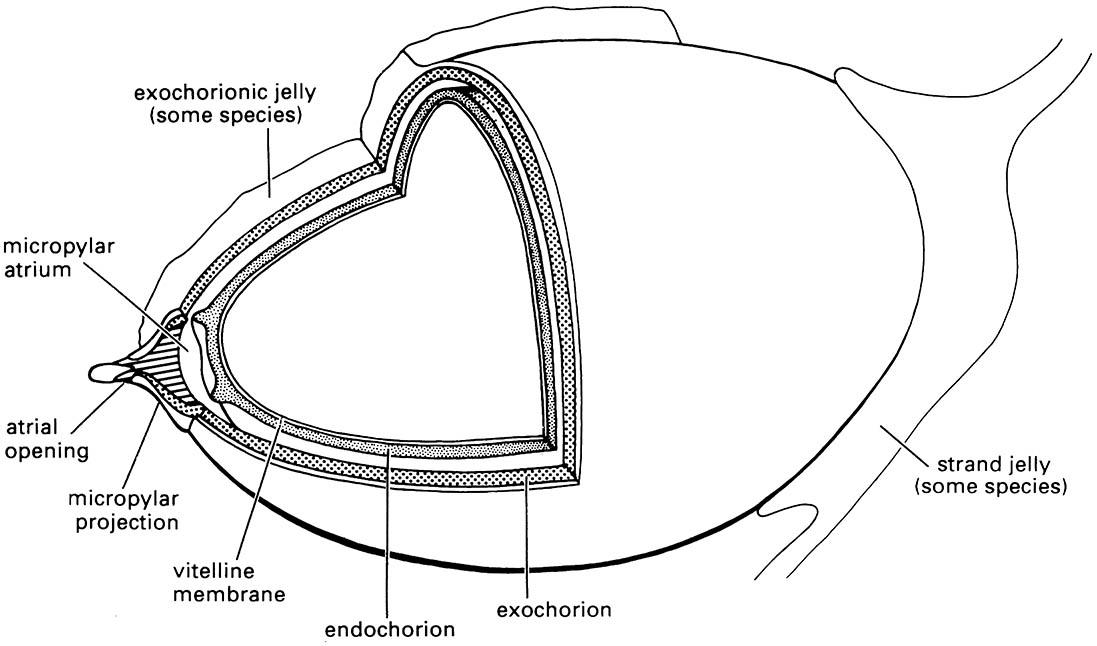
Libelluloid dragonflies oviposit into freshwater but always exophytically (i.e. outside of plant tissues). The endochorionic and exochorionic layers of the eggshell are separated by a distinct gap in some species. A gelatinous matrix may be present on the exochorion or as connecting strands between eggs. (After Trueman 1991)
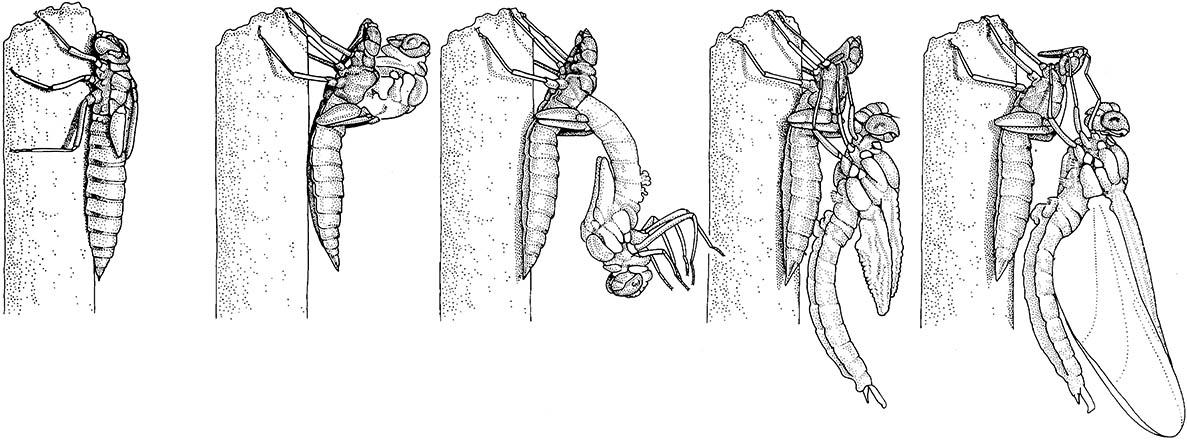
The final-instar nymph climbs out of the water prior to the shedding of its cuticle. The old cuticle splits