13.1.2. Active foraging
More energetic foraging involves active searching for suitable patches, and once there, for prey or for hosts. Movements associated with foraging and with other locomotory activities, such as seeking a mate, are so similar that the “motivation” may be recognized only in retrospect, by resultant prey capture or host finding. The locomotory search patterns used to locate prey or hosts are those described for general orientation in section 4.5, and comprise non-directional (random) and directional (non-random) locomotion.
Random, or non-directional foraging
The foraging of aphidophagous larval coccinellid beetles and syrphid flies amongst their clumped prey illustrates several features of random food searching. The larvae advance, stop periodically, and “cast” about by swinging their raised anterior bodies from side to side. Subsequent behavior depends upon whether or not an aphid is encountered. If no prey is encountered, motion continues, interspersed with casting and turning at a fundamental frequency. However, if contact is made and feeding has taken place or if the prey is encountered and lost, searching intensifies with an enhanced frequency of casting, and, if the larva is in motion, increased turning or direction-changing. Actual feeding is unnecessary to stimulate this more concentrated search: an unsuccessful encounter is adequate. For early-instar larvae that are very active but have limited ability to handle prey, this stimulus to search intensively near a lost feeding opportunity is important to survival.
Most laboratory-based experimental evidence, and models of foraging based thereon, are derived from single species of walking predators, frequently assumed to encounter a single species of prey randomly distributed within selected patches. Such premises may be justified in modeling grossly simplified ecosystems, such as an agricultural monoculture with a single pest controlled by one predator. Despite the limitations of such laboratory-based models, certain findings appear to have general biological relevance.
An important consideration is that the time allocated to different patches by a foraging predator depends upon the criteria for leaving a patch. Four mechanisms have been recognized to trigger departure from a patch:
- a certain number of food items have been encountered (fixed number);
- a certain time has elapsed (fixed time);
- a certain searching time has elapsed (fixed searching time);
- the prey capture rate falls below a certain threshold (fixed rate).
The fixed-rate mechanism has been favored by modelers of optimal foraging, but even this is likely to be a simplification if the forager’s responsiveness to prey is non-linear (e.g. declines with exposure time) and/or derives from more than simple prey encounter rate, or prey density. Differences between predator—prey interactions in simplified laboratory conditions and the actuality of the field cause many problems, including failure to recognize variation in prey behavior that results from exposure to predation (perhaps multiple predators). Furthermore, there are difficulties in interpreting the actions of polyphagous predators, including the causes of predator/parasitoid/parasite behavioral switching between different prey animals or hosts.
Non-random, or directional foraging
Several more specific directional means of host finding can be recognized, including the use of chemicals, sound, and light. Experimentally these are rather difficult to establish, and to separate, and it may be that the use of these cues is very widespread, if little understood. Of the variety of cues available, many insects probably use more than one, depending upon distance or proximity to the resource sought. Thus, the European crabronid wasp Philanthus, which eats only bees, relies initially on vision to locate moving insects of appropriate size. Only bees, or other insects to which bee odors have been applied experimentally, are captured, indicating a role for odor when near the prey. However, the sting is applied only to actual bees, and not to bee-smelling alternatives, demonstrating a final tactile recognition.
Not only may a stepwise sequence of stimuli be necessary, as seen above, but also appropriate stimuli may have to be present simultaneously in order to elicit appropriate behavior. Thus, Telenomus heliothidis (Hymenoptera: Scelionidae), an egg parasitoid of Heliothis virescens (Lepidoptera: Noctuidae), will investigate and probe at appropriate-sized round glass beads that emulate Heliothis eggs, if they are coated with female moth proteins. However, the scelionid makes no response to glass beads alone, or to female moth proteins applied to improperly shaped beads.
Chemicals
The world of insect communication is dominated by chemicals, or pheromones (section 4.3). Ability to detect the chemical odors and messages produced by prey or hosts (kairomones) allows specialist predators and parasitoids to locate these resources. Certain para- sitic tachinid flies and braconid wasps can locate their respective stink bug or coccoid host by tuning to their hosts’ long-distance sex attractant pheromones. Several unrelated parasitoid hymenopterans use the aggregation pheromones of their bark and timber beetle hosts. Chemicals emitted by stressed plants, such as terpenes produced by pines when attacked by an insect, act as synomones (communication chemicals that benefit both producer and receiver); for example, certain pteromalid (Hymenoptera) parasitoids locate their hosts, the damage-causing scolytid timber beetles, in this way. Some species of tiny wasps (Trichogrammatidae) that are egg endoparasitoids (Fig. 16.3) are able to locate the eggs laid by their preferred host moth by the sex attractant pheromones released by the moth. Furthermore, there are several examples of parasitoids that locate their specific insect larval hosts by “frass” odors — the smells of their feces. Chemical location is particularly valuable when hosts are concealed from visual inspection, for example when encased in plant or other tissues.
Chemical detection need not be restricted to tracking volatile compounds produced by the prospective host. Thus, many parasitoids searching for phytophagous insect hosts are attracted initially, and at a distance, to host-plant chemicals, in the same manner that the phytophage located the resource. At close range, chemicals produced by the feeding damage and/or frass of phytophages may allow precise targeting of the host. Once located, the acceptance of a host as suitable is likely to involve similar or other chemicals, judging by the increased use of rapidly vibrating antennae in sensing the prospective host.
Blood-feeding adult insects locate their hosts using cues that include chemicals emitted by the host. Many female biting flies can detect increased carbon dioxide levels associated with animal respiration and fly upwind towards the source. Highly host-specific biters probably also are able to detect subtle odors: thus, human-biting black flies (Diptera: Simuliidae) respond to components of human exocrine sweat glands. Both sexes of tsetse flies (Diptera: Glossinidae) track the odor of exhaled breath, notably carbon dioxide, octanols, acetone, and ketones emitted by their preferred cattle hosts.
Sound
The sound signals produced by animals, including those made by insects to attract mates, have been utilized by some parasites to locate their preferred hosts acoustically. Thus, the blood-sucking females of Corethrella (Diptera: Corethrellidae) locate their favored host, hylid treefrogs, by following the frogs’ calls. The details of the host-finding behavior of ormiine tachinid flies are considered in detail in Box 4.1. Flies of two other dipteran species are known to be attracted by the songs of their hosts: females of the larviparous tachinid Euphasiopteryx ochracea locate the male crickets of Gryllus integer, and the sarcophagid Colcondamyia auditrix finds its male cicada host, Okanagana rimosa, in this manner. This allows precise deposition of the parasitic immature stages in, or close to, the hosts in which they are to develop.
Predatory biting midges (Ceratopogonidae) that prey upon swarm-forming flies, such as midges (Chironomidae), appear to use cues similar to those used by their prey to locate the swarm; cues may include the sounds produced by wing-beat frequency of the members of the swarm. Vibrations produced by their hosts can be detected by ectoparasites, notably amongst the fleas. There is also evidence that certain parasitoids can detect at close range the substrate vibration produced by the feeding activity of their hosts. Thus, Biosteres longicaudatus, a braconid hymenopteran endoparasitoid of a larval tephritid fruit fly (Diptera: Anastrepha suspensa), detects vibrations made by the larvae moving and feeding within fruit. These sounds act as a behavioral releaser, stimulating host-finding behavior as well as acting as a directional cue for their concealed hosts.
Light
The larvae of the Australian cave-dwelling mycetophilid fly Arachnocampa and its New Zealand counterpart, Bolitophila luminosa, use bioluminescent lures to catch small flies in sticky threads that they suspend from the cave ceiling. Luminescence (section 4.4.5), as with all communication systems, provides scope for abuse; in this case, the luminescent courtship signaling between beetles is misappropriated. Carnivorous female lampyrids of some Photurus species, in an example of aggressive foraging mimicry, can imitate the flashing signals of females of up to five other firefly species. The males of these different species flash their responses and are deluded into landing close by the mimetic female, whereupon she devours them. The mimicking Photurus female will eat the males of her own species, but can- nibalism is avoided or reduced as the Photurus female is most piratical only after mating, at which time she becomes relatively unresponsive to the signals of males of her own species.
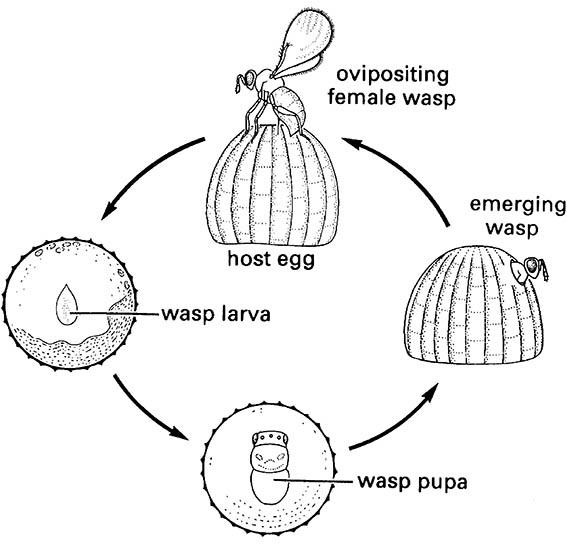
A tiny female wasp of a Trichogramma species (Hymenoptera: Trichogrammatidae) oviposits into a lepidopteran egg; the wasp larva develops within the host egg, pupates, and emerges as an adult, often with the full life cycle taking only one week. (After van den Bosch & Hagen 1966)

