13.1.1. Sitting and waiting
Sit-and-wait predators find a suitable patch and wait for mobile prey to come within striking range. As the vision of many insects limits them to recognition of movement rather than precise shape, a sit-and-wait predator may need only to remain motionless in order to be unobserved by its prey. Nonetheless, amongst those that wait, many have some form of camouflage (crypsis). This may be defensive, being directed against highly visual predators such as birds, rather than evolved to mislead invertebrate prey. Cryptic predators modeled on a feature that is of no interest to the prey (such as tree bark, lichen, a twig, or even a stone) can be distinguished from those that model on a feature of some significance to prey, such as a flower that acts as an insect attractant.
In an example of the latter case, the Malaysian mantid Hymenopus bicornis closely resembles the red flowers of the orchid Melastoma polyanthum amongst which it rests. Flies are encouraged to land, assisted by the presence of marks resembling flies on the body of the mantid: larger flies that land are eaten by the mantid. In another related example of aggressive foraging mimicry, the African flower-mimicking mantid Idolum does not rest hidden in a flower, but actually resembles one due to petal-shaped, colored outgrowths of the pro- thorax and the coxae of the anterior legs. Butterflies and flies that are attracted to this hanging “flower” are snatched and eaten.
Ambushers include cryptic, sedentary insects such as mantids, which prey fail to distinguish from the inert, non-floral plant background. Although these predators rely on the general traffic of invertebrates associated with vegetation, often they locate close to flowers, to take advantage of the increased visiting rate of flower feeders and pollinators.
Odonate nymphs, which are major predators in many aquatic systems, are classic ambushers. They rest concealed in submerged vegetation or in the substrate, waiting for prey to pass. These predators may show dual strategies: if waiting fails to provide food, the hungry insect may change to a more active searching mode after a fixed period. This energy expenditure may bring the predator into an area of higher prey density. In running waters, a disproportionately high number of organisms found drifting passively with the current are predators: this drift constitutes a low-energy means for sit-and-wait predators to relocate, induced by local prey shortage.
Sitting-and-waiting strategies are not restricted to cryptic and slow-moving predators. Fast-flying, diurnal, visual, rapacious predators such as many robber flies (Diptera: Asilidae) and adult odonates spend much time perched prominently on vegetation. From these conspicuous locations their excellent sight allows them to detect passing flying insects. With rapid and accurately controlled flight, the predator makes only a short foray to capture appropriately sized prey. This strategy combines energy saving, through not needing to fly incessantly in search of prey, with time efficiency, as prey is taken from outside the immediate area of reach of the predator.
Another sit-and-wait technique involving greater energy expenditure is the use of traps to ambush prey. Although spiders are the prime exponents of this method, in the warmer parts of the world the pits of certain larval antlions (Neuroptera: Myrmeleontidae) (Fig. 13.2a,b) are familiar. The larvae either dig pits directly or form them by spiraling backwards into soft soil or sand. Trapping effectiveness depends upon the steepness of the sides, the diameter, and the depth of the pit, which vary with species and instar. The larva waits, buried at the base of the conical pit, for passing prey to fall in. Escape is prevented physically by the slip-periness of the slope, and the larva may also flick sand at prey before dragging it underground to restrict its defensive movements. The location, construction, and maintenance of the pit are vitally important to capture efficiency but construction and repair is energetically very expensive. Experimentally it has been shown that even starved Japanese antlions (Myrmeleon bore) would not relocate their pits to an area where prey was provided artificially. Instead, larvae of this species of antlion reduce their metabolic rate to tolerate famine, even if death by starvation is the result.
In holometabolous ectoparasites, such as fleas and parasitic flies, immature development takes place away from their vertebrate hosts. Following pupation, the adult must locate the appropriate host. Since in many of these ectoparasites the eyes are reduced or absent, vision cannot be used. Furthermore, as many of these insects are flightless, mobility is restricted. In fleas and some Diptera, in which larval development often takes place in the nest of a host vertebrate, the adult insect waits quiescent in the pupal cocoon until the presence of a host is detected. The duration of this quiescent period may be a year or longer, as in the cat flea (Ctenocephalides felis) — a familiar phenomenon to humans that enter an empty dwelling that previously housed flea-infested cats. The stimuli to cease dormancy include some or all of: vibration, rise in temperature, increased carbon dioxide, or another stimulus generated by the host.
In contrast, the hemimetabolous lice spend their lives entirely on a host, with all developmental stages ectoparasitic. Any transfer between hosts is either through phoresy (see below) or when host individuals make direct contact, as from mother to young within a nest.
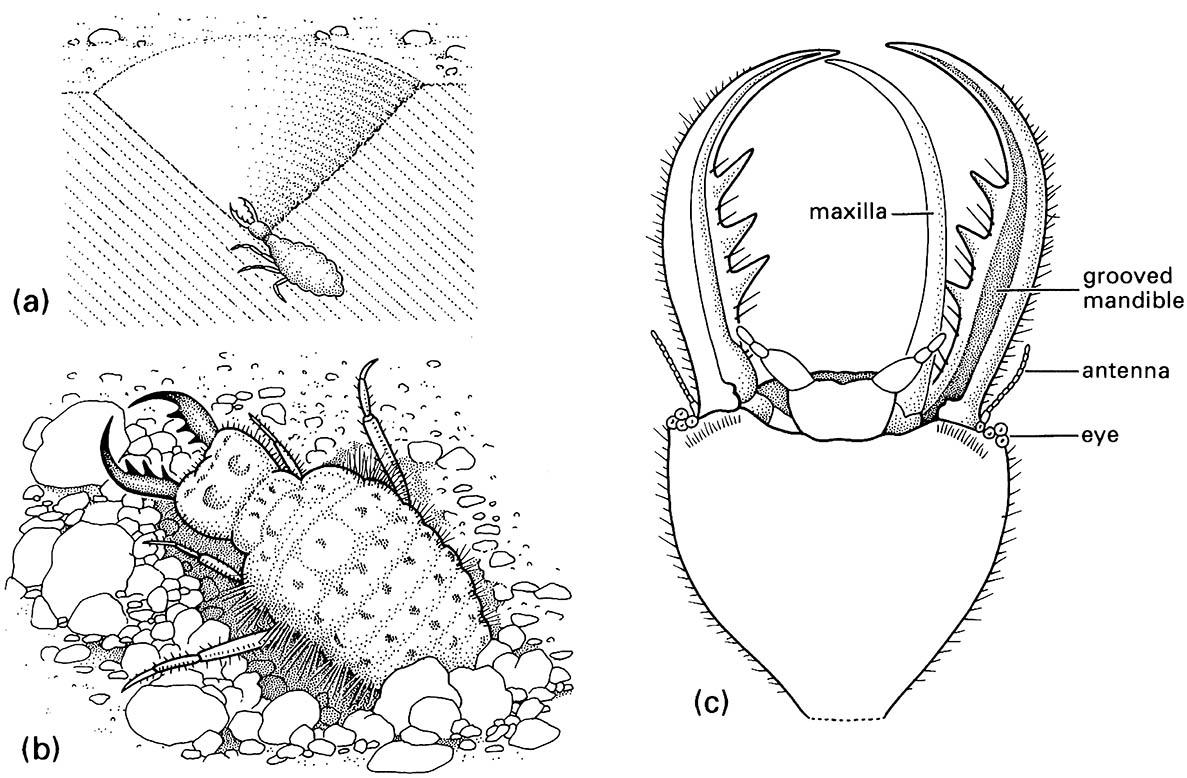
(a) larva in its pit in sand; (b) detail of dorsum of larva; (c) detail of ventral view of larval head showing how the maxilla fits against the grooved mandible to form a sucking tube. (After Wigglesworth 1964)

