13.3.3. Patterns of host use and specificity in parasites
The wide array of insects that are ectoparasitic upon vertebrate hosts are of such significance to the health of humans and their domestic animals that we devote a complete chapter to them (Chapter 15) and medical issues will not be considered further here. In contrast to the radiation of ectoparasitic insects using vertebrate hosts and the immense numbers of species of insect parasitoids seen above, there are remarkably few insect parasites of other insects, or indeed, of other arthropods.
The largest group of endoparasitic insects using other insects as hosts belongs to the Strepsiptera, an order comprising a few hundred exclusively parasitic species (Box 13.6). The characteristically aberrant bodies of their predominantly hemipteran and hyme- nopteran hosts are termed “stylopized”, so-called for a common strepsipteran genus, Stylops. Within the host’s body cavity, growth of larvae and pupae of both sexes, and the adult female strepsipteran, causes malformations including displacement of the internal organs. The host’s sexual organs degenerate, or fail to develop appropriately.
Although larval Dryinidae (Hymenoptera) develop parasitically part-externally and part-internally in hemipterans, virtually all other insect—insect parasitic interactions involve ectoparasitism. The Braulidae is a family of Diptera comprising some aberrant, mite-like flies belonging to a single genus, Braula, intimately associated with Apis (honey bees). Larval braulids scavenge on pollen and wax in the hive, and the adults usurp nectar and saliva from the proboscis of the bee. This association certainly involves phoresy, with adult braulids always found on their hosts’ bodies, but whether the relationship is ectoparasitic is open to debate. Likewise, the relationship of several genera of aquatic chironomid larvae with nymphal hosts, such as mayflies, stoneflies, and dragonflies, ranges from phoresy to suggested ectoparasitism. Generally, there is little evidence that any of these ecto- and endoparasites using insects show a high degree of specificity at the species level. However, this is not necessarily the case for insect parasites with vertebrate hosts.
The patterns of host-specificity and preferences of parasites raise some of the most fascinating questions in parasitology. For example, most orders of mammals bear lice (Phthiraptera), many of which are monoxenic or found amongst a limited range of hosts. Even some marine mammals, namely certain seals, have lice, although whales do not. No Chiroptera (bats) harbor lice, despite their apparent suitability, although they host many other ectoparasitic insects, including the Streblidae and Nycteribiidae — two families of ectoparasitic Diptera that are restricted to bats.
Some terrestrial hosts are free of all ectoparasites, others have very specific associations with one or a few guests, and in Panama the opossum Didelphis marsupialis has been found to harbor 41 species of ectoparasitic insects and mites. Although four or five of these are commonly present, none are restricted to the opossum and the remainder are found on a variety of hosts, ranging from distantly related mammals to reptiles, birds, and bats.
We can examine some principles concerning the different patterns of distribution of parasites and their hosts by looking in some detail at cases where close associations of parasites and hosts are expected. The findings can then be related to ectoparasite—host relations in general.
The Phthiraptera are obligate permanent ectopara- sites, spending all their lives on their hosts, and lacking any free-living stage. Extensive surveys, such as one which showed that neotropical birds averaged 1.1 lice species per host across 127 species and 26 families of birds, indicate that lice are highly monoxenous (restricted to one host species). A high level of coevolution between louse and host might be expected, and in general, related animals have related lice. The widely quoted Fahrenholz’s rule formally states that the phylogenies of hosts and parasites are identical, with every speciation event affecting hosts being matched by a synchronous speciation of the parasites, as shown in Fig. 13.8a.
It follows that:
- phylogenetic trees of hosts can be derived from the trees of their ectoparasites;
- ectoparasite phylogenetic trees are derivable from the trees of their hosts (the potential for circularity of reasoning is evident);
- the number of parasite species in the group under consideration is identical to the number of host species considered;
- no species of host has more than one species of parasite in the taxon under consideration;
- no species of parasite parasitizes more than one species of host.
Fahrenholz’s rule has been tested for mammal lice selected from amongst the family Trichodectidae, for which robust phylogenetic trees, derived independently of any host mammal phylogeny, are available. Amongst a sample of these trichodectids, 337 lice species parasitize 244 host species, with 34% of host species parasitized by more than one trichodectid. Several possible explanations exist for these mismatches. Firstly, speciation may have occurred independently amongst certain lice on a single host (Fig. 13.8b). This is substantiated, with at least 7% of all speciation events in the sampled Trichodectidae showing this pattern of independent speciation. A second explanation involves secondary transfer of lice species to phylogenetically unrelated host taxa. Amongst extant species, when cases arising from human-induced unnatural host proximity are excluded (accounting for 6% of cases), unmistakable and presumed natural transfers (i.e. between marsupial and eutherian mammal, or bird and mammal) occur in about 2% of speciation events. However, hidden within the phylogenies of host and parasite are speciation events that involve lateral transfer between rather more closely-related host taxa, but these transfers fail to match precisely the phylogeny. Examination of the detailed phylogeny of the sampled Trichodectidae shows that a minimum of 20% of all speciation events are associated with distant and lateral secondary transfer, including historical transfers (lying deeper in the phylogenetic trees).
In detailed examinations of relationships between a smaller subset of trichodectids and eight of their pocket gopher (Rodentia: Geomyidae) hosts, substantial concordance was claimed between trees derived from biochemical data for hosts and parasites, and some evidence of co-speciation was found. However, many of the hosts were shown to have two lice species, and unconsidered data show most species of gopher to have a substantial suite of associated lice. Furthermore, a minimum of three instances of lateral transfer (host switching) appeared to have occurred, in all cases between hosts with geographically contiguous ranges. Although many speciation events in these lice “track” speciation in the host and some estimates even indicate similar ages of host and parasite species, it is evident from the Trichodectidae that strict co-speciation of host and parasite is not the sole explanation of the associations observed.
The reasons why apparently monoxenic lice sometimes do deviate from strict coevolution and co- speciation apply equally to other ectoparasites, many of which show similar variation in complexity of host relationships. Deviations from strict co-speciation arise if host speciation occurs without commensurate para- site speciation (Fig. 13.8c). This resulting pattern of relationships is identical to that seen if one of two parasite sister taxa generated by co-speciation in concert with the host subsequently became extinct. Frequently, a parasite is not present throughout the complete range of its host, resulting perhaps from the parasite being restricted in range by environmental factors independent of those controlling the range of the host. Hemimetabolous ectoparasites, such as lice, which spend their entire lives on the host, might be expected to closely follow the ranges of their hosts, but there are exceptions in which the ectoparasite distribution is restricted by external environmental factors. For holometabolous ectoparasites, which spend some of their lives away from their hosts, such external factors will be even more influential in governing para- site range. For example, a homeothermic vertebrate may tolerate environmental conditions that cannot be sustained by the free-living stage of a poikilothermic ectoparasite, such as a larval flea. As speciation may occur in any part of the distribution of a host, host speciation may be expected to occur without necessarily involving the parasite. Furthermore, a parasite may show geographical variation within all or part of the host range that is incongruent with the variation of the host. If either or both variations lead to eventual species formation, there will be incongruence between parasite and host phylogeny.
Furthermore, poor knowledge of host and parasite interactions may result in misleading conclusions. A true host may be defined as one that provides the conditions for parasite reproduction to continue indefinitely. When there is more than one true host, there may be a principal (preferred) or exceptional host, depending on the proportional frequencies of ectoparasite occurrence. An intermediate category may be recognized — the sporadic or secondary host — on which parasite development cannot normally take place, but an association arises frequently, perhaps through predator— prey interactions or environmental encounters (such as a shared nest). Small sample sizes and limited biological information can allow an accidental or secondary host to be mistaken for a true host, giving rise to a possible erroneous “refutation” of co-speciation. Extinctions of certain parasites and true hosts (leaving the parasite extant on a secondary host) will refute Fahrenholz’s rule.
Even assuming perfect recognition of true host- specificity and knowledge of the historical existence of all parasites and hosts, it is evident that successful parasite transfers between hosts have taken place throughout the history of host—parasite interactions. Co-speciation is fundamental to host—parasite relations, but the factors encouraging deviations must be considered. Predominantly, these concern (i) geographical and social proximity of different hosts, allowing opportunities for parasite colonization of the new host, together with (ii) ecological similarity of different hosts, allowing establishment, survival, and reproduction of the ectoparasite on the novel host. The results of these factors have been termed resource tracking, to contrast with the phyletic tracking implied by Fahrenholz’s rule. As with all matters biological, most situations lie somewhere along a continuum between these two extremes, and rather than forcing patterns into one category or the other, interesting questions arise from recognizing and interpreting the different patterns observed.
If all host—parasite relationships are examined, some of the factors that govern host-specificity can be identified:
- the stronger the life-history integration with that of the host, the greater the likelihood of monoxeny;
- the greater the vagility (mobility) of the parasite, the more likely it is to be polyxenous;
- the number of accidental and secondary parasite species increases with decreasing ecological specialization and with increase in geographical range of the host, as we saw earlier in this section for the opossum, which is widespread and unspecialized.
If a single host shares a number of ectoparasites, there may be some ecological or temporal segregation on the host. For example, in hematophagous (blood-sucking) black flies (Simuliidae) that attack cattle, the belly is more attractive to certain species, whereas others feed only on the ears. Pediculus humanus capitis and P. humanus corporis (Phthiraptera), human head and body lice respectively, are ecologically separated examples of sibling taxa in which strong reproductive isolation is reflected by only slight morphological differences.
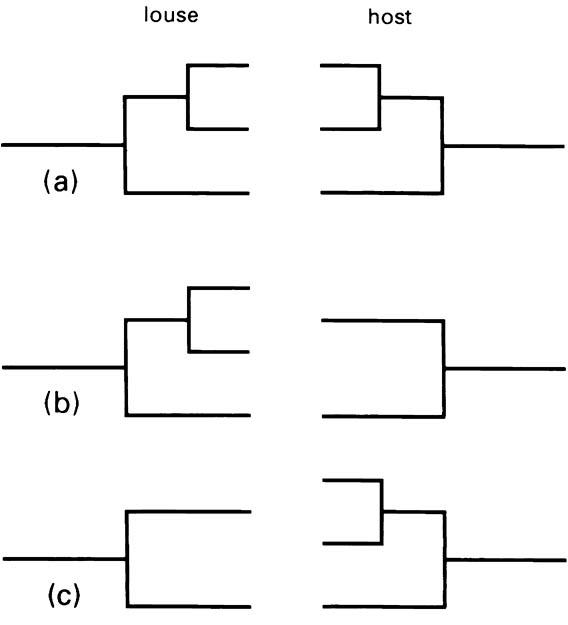
(a) adherence to Fahrenholz’s rule; (b) independent speciation of the lice; (c) independent speciation of the hosts. (After Lyal 1986)

