8.2.1. The insect fossil record
Until recently, the oldest fossil hexapods were Collembola, including Rhyniella praecursor, known from about 400 mya (million years ago) in the Lower Devonian of Rhynie, Scotland, and slightly younger archaeognathans from North America (Fig. 8.1). Reinterpretation of another Rhynie fossil, Rhyniognatha hirsti, known only from its mouthparts, suggests that it is the oldest “ectognathous” insect. Tantalizing evidence from Lower Devonian fossil plants shows damage resembling that caused by the piercing-and-sucking mouthparts of insects or mites. Any earlier fossil evidence for Insecta or their relatives will be difficult to find because appropriate freshwater fossiliferous deposits are scarce prior to the Devonian.
In the Carboniferous, an extensive radiation is evidenced by substantial Upper Carboniferous fossils. Lower Carboniferous fossils are unknown, again because of lack of freshwater deposits. By some 300 mya a probably monophyletic grouping of Palaeodictyopteroidea comprising three now-extinct ordinal groups, the Megasecoptera, Palaeodictyoptera (Fig. 8.2), and Diaphanopterodea, was diverse. Palaeodictyopteroideans varied in size (with wingspans up to 56 cm), diversity (over 70 genera in 21 families are known), and in morphology, notably in mouthparts and wing articulation and venation. An apparently paraphyletic “Protodonata”, perhaps a stemgroup of Odonata, had prothorax winglets as did Palaeodictyopteroidea, and included Permian insects with the largest wingspans ever recorded. Extant orders represented unambiguously by Carboniferous fossils are limited to the Orthoptera; putative Ephemeroptera, fossil hemipteroids, and blattoids are treated best as paraphyletic groups lacking the defining features of any extant clade. The beak-like, piercing mouthparts and expanded clypeus of some Carboniferous insects indicate an early origin of plant-feeding, although it was not until the Permian that gymnosperms (conifers and allies) became abundant in the previously fern-dominated flora. Concurrently, a dramatic increase took place in ordinal diversity, with some 30 orders known from the Permian. The evolution of the plant-sucking Hemiptera may have been associated with the newly available plants with thin cortex and sub-cortical phloem. Other Permian insects included those that fed on pollen, another resource of previously restricted supply.
Certain Carboniferous and Permian insects were very large, exemplified by the giant Bolsover dragonfly and palaeodictyopteran on a Psaronius tree fern, depicted in the vignette for this chapter. Fossils of some Ephemeroptera and dragonfly-like Protodonata had wingspans of up to 45 cm and 71 cm respectively. A plausible explanation for this gigantism is that the respiratory restriction on insect size (section 3.5.1) might have been alleviated by elevated atmospheric oxygen levels during the late Palaeozoic (between 370 and 250 mya), which would have promoted greater oxygen diffusion in the tracheae. Furthermore, if other gases were unchanged, then any extra atmospheric oxygen may have facilitated flight in denser air. Despite the obvious appeal of this hypothesis to account for Palaeozoic gigantism, such a climatic reconstruction remains controversial and the physiological and morphological consequences of alterations of gaseous composition have been little studied for insects.
Many groups present in the Permian, including Ephemeroptera, Odonata, Plecoptera, Grylloblattodea, Phasmatodea, Orthoptera, Dictyoptera and other Polyneoptera, plus Coleoptera and Neuropterida amongst other early originating Holometabola, survived the period. However, early lineages such as Palaeodictyopteroidea (including Diaphanopteroidea, Megasecoptera, and Palaeodictyoptera) disappeared at the end of the Permian. This Permian—Triassic boundary was a time of major extinction that particularly affected marine biota, and coincided with a dramatic reduction in diversity in taxa and feeding types within surviving insect orders.
The Triassic period (commencing about 245 mya) is famed for the “dominance” by dinosaurs and pterosaurs, and the origin of the mammals; but the insects were radiating apace. The major orders of modern insects, excepting Hymenoptera and Lepidoptera, were well established by the beginning of the Triassic. Hymenoptera are seen first in this period, but represented only by symphytans. The oldest living families appeared, together with diversified taxa with aquatic immature (and some adult) stages, including modern Odonata, Heteroptera, and many families of nematocerous Diptera. The Jurassic saw the first appearance of aculeate Hymenoptera, many early-branching Diptera, and the first Brachycera. Triassic and Jurassic fossils include some excellent preserved material in fine- grained deposits such as those of Solenhofen, the site of beautifully preserved insects and Archaeopteryx. The origin of birds (Aves) and their subsequent diversification marked the first aerial competition for insects since the evolution of flight.
In the Cretaceous (145–65 mya) and throughout the subsequent Tertiary period (65–1.6 mya), excel- lent arthropod specimens were preserved in amber — a resinous plant secretion that trapped insects and hardened into a clear preservative (see Plate 3.6). The excellence of preservation of whole insects in amber contrasts favorably with compression fossils that may comprise little more than crumpled wings. Many early fossil records of extant higher taxa (groups above species level) derive from these well-preserved amber specimens, but inherent sampling biases must be recognized. Smaller (more easily trapped) and forest-dwelling taxa are over-represented. Amber of Cretaceous origin occurs in France, Lebanon, Burma, Siberia, Canada, and New Jersey. The biota of this period shows a numerical dominance of insects coincident with angiosperm (flowering plant) diversification. However, major mouthpart types of extant insects evolved prior to the angiosperm radiation, associated with insect feeding on earlier terrestrial plant radiations. The fossil record indicates the great antiquity of certain insect—plant associations. The lower Cretaceous of China (130 mya) has revealed both early angiosperms and a distinctive fly belonging to Nemestrinidae with a characteristic long proboscis associated with angiosperm pollination. Elsewhere, a fossil leaf of an ancestral sycamore has the highly characteristic mine of the extant genus Ectoedemia (Lepidoptera: Nepticulidae), suggesting at least a 97 million year association between the nepticulid moth and particular plants. Both Coleoptera and Lepidoptera, which are primarily phytophagous orders, commenced their massive radiations in the Cretaceous. By 65 mya, the insect fauna looks rather modern, with some fossils able to be allocated to extant genera. For many animals, notably the dinosaurs, the Cretaceous—Tertiary (“K—T”) boundary marked a major extinction event. Although it is generally believed that the insects entered the Tertiary with little extinction, recent studies show that although generalized insect—plant interactions survived, the prior high diversity of specialist insect—plant associations was greatly attenuated. At least in the paleobiota of south-western North Dakota, at 65 mya a major ecological perturbation setback specialized insect—plant associations.
Our understanding of Tertiary insects increasingly comes from amber from the Dominican Republic (see Plate 3.6) dated to the Oligocene/Miocene (37–22 mya) to complement the abundant and well-studied Baltic amber that derives from Eocene/Oligocene deposits (50–30 mya). Baltic ambers have been preserved and are now partially exposed beneath the northern European Baltic Sea and, to a lesser extent, the southern North Sea, brought to shore by periodic storms. Many attempts have been made to extract, amplify, and sequence ancient DNA from fossil insects preserved in amber — an idea popularized by the movie Jurassic Park. Amber resin is argued to dehydrate specimens and thus protect their DNA from bacterial degradation. Success in sequencing ancient DNA has been claimed for a variety of amber-preserved insects, including a termite (30 myo (million years old)), a stingless bee (25–40 myo), and a weevil (120–135 myo); however, attempts to authenticate these ancient sequences by repetition have failed. Degradation of DNA from amber fossils and contamination by fresh DNA are unresolved issues, but the extraction of material from amber insects leads to certain destruction of the specimen. Even if authentic DNA sequences have been obtained from amber insects, as yet they do not provide more useful information than that which derives from the often exquisitely preserved morphology.
An unchallengeable outcome of recent studies of fossil insects is that many species and higher taxa, especially genera and families, are revealed as much older than thought previously. At species level, all northern temperate, sub-Arctic, and Arctic zone fossil insects dating from the last million years or so appear to be morphologically identical to existing species. Many of these fossils belong to beetles (particularly their elytra), but the situation seems no different amongst other insects. Pleistocene climatic fluctuations (glacial and interglacial cycles) evidently caused taxon range changes, via movements and extinctions of individuals, but resulted in the genesis of few, if any, new species, at least as defined on their morphology. The implication is that if species of insect are typically greater than a million years old, then insect higher taxa such as genera and families may be of immense age.
Modern microscopic paleontological techniques can allow inference of age for insect taxa based on recognition of specific types of feeding damage caused to plants which are fossilized. As seen above, leaf mining in ancient sycamore leaves is attributable to a genus of extant nepticulid moth, despite the absence of any preserved remains of the actual insect. In like manner, a hispine beetle (Chrysomelidae) causing a unique type of grazing damage on young leaves of ginger plants (Zingiberaceae) has never been seen preserved as contemporary with the leaf fossils. The characteristic damage caused by their leaf chewing is recognizable, however, in the Late Cretaceous (c. 65 mya) deposits from Wyoming, some 20 million years before any body fossil of the culprit hispine. To this day, these beetles specialize in feeding on young leaves of gingers and heliconias of the modern tropics.
Despite these valuable contributions made by fossils, several tempting inferences concerning their use in phylogenetic reconstruction ought to be resisted, namely that:
- all character states in the fossil are in the ancestral condition;
- any fossil represents an actual ancestral form of later taxa;
- the oldest fossil of a group necessarily represents the phylogenetically earliest taxon.
Notwithstanding this last point, fossil insects tend to demonstrate that the stratigraphic (time) sequence of earliest-dated fossils reflects the branching sequence of the phylogeny. Early-branching (so-called “basal”) taxa within each order are represented first — for example, the Mastotermitidae before the higher termites (Fig. 7.4), midges, gnats, and sand flies before house and blow flies, and primitive moths before the butterflies (Fig. 7.7). Furthermore, insect fossils can show that taxa currently restricted (or perhaps disjunct) in distribution once were distributed more widely. Some such taxa include the following.
- Mastotermitidae (Isoptera), now represented by one northern Australian species, is diverse and abundant in ambers from the Dominican Republic (Caribbean), Brazil, Mexico, USA, France, Germany, and Poland dating from Cenomanian Cretaceous (c. 100 mya) to lower Miocene (some 20 mya);
- the biting-midge subfamily Austroconopinae (Diptera: Ceratopogonidae), now restricted to one extant species of Austroconops in Western Australia, is represented by several species in Lower Cretaceous Lebanese amber (Neocomian, 120 mya) and Upper Cretaceous Siberian amber (90 mya);
- Leptomyrmex, an ant genus now distributed in the western Pacific (eastern Australia, New Guinea, New Caledonia), is known from 30–40 myo Dominican amber.
An emerging pattern stemming from ongoing study of amber insects, especially those dating from the Cretaceous, is the former presence in the north of groups now restricted to the south. We might infer that the modern distributions, often involving Australia, are relictual due to differential extinction in the north. The question of whether such patterns relate in any way to northern extinction at the K—T boundary due to bolide (“meteorite”) impact deserves further study. Did the differential extinction of specialist insect—plant associations in South Dakota (above) extend to the southern hemisphere? Evidently, some insect taxa presently restricted to the southern hemisphere but reported as having been present 30–40 mya in Dominican and subsequent Baltic ambers did survive the event and regional extinction has occurred more recently.
The relationship of fossil insect data to phylogeny derivation is complex. Although early fossil taxa seem often to precede phylogenetically later-branching (“more derived”) taxa, it is methodologically unsound to assume so. Although phylogenies can be reconstructed from the examination of characters observed in extant material alone, fossils provide important information, not least allowing dating of the minimum age of origin of diagnostic derived characters and clades. Optimally, all data, fossil and extant, can and should be reconciled into a single estimate of evolutionary history.
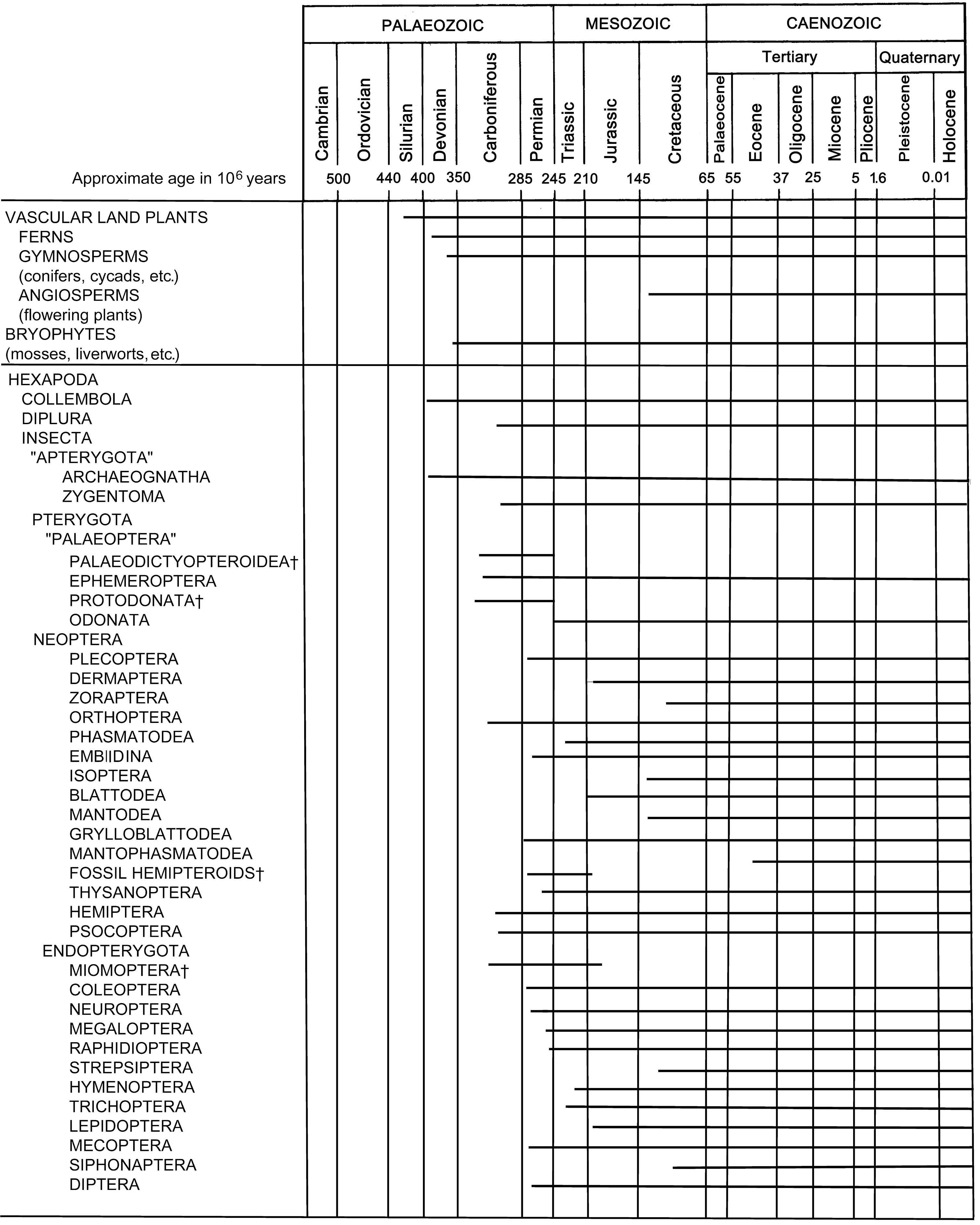
Taxa that contain only fossils are indicated by the symbol †. The fossil record of Protura, Phthiraptera, and Zoraptera dates from only the Holocene, presumably because of the sma ll size and/or rarity of these organisms. Miomoptera represent the earliest fossil endopterygotes. The placement of Rhyniognatha is unknown. (Insect records after Kukalová-Peck 1991; and Conrad Labandeira (Smithsonian Institution) pers. comm.)
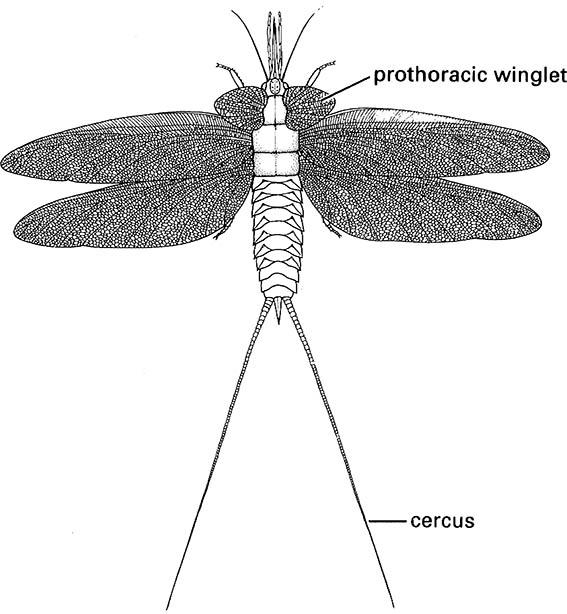
(After Kukalová 1970)

The broken line indicates a paraphyletic taxon. (Data from several sources)
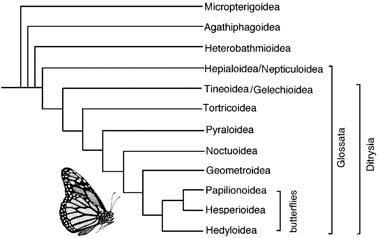
(After Kristensen & Skalski 1999)

