8.7. Insect evolution in the Pacific
Study of the evolution of insects (and other arthropods such as spiders) of oceanic islands such as Hawai’i and the Galapagos is comparable in importance to those of the perhaps more famous plants (e.g. Hawai’an silverswords), birds (Hawai’ian honeycreepers and “Darwin’s finches” of the Galapagos), land snails (Hawai’i), and lizards (Galapagos iguanas). The earliest and most famous island evolutionary studies of insects involved the Hawai’ian fruit flies (Diptera: Drosophilidae). This radiation has been revisited many times, but recent research has included evolutionary studies of certain crickets, microlepidopterans, carabid beetles, pipunculid flies, mirid bugs, and damselflies.
Why this interest in the fauna of isolated island chains in the mid-Pacific? The Hawai’ian fauna is highly endemic, with an estimated 99% of its native arthropod species found nowhere else. The Pacific is an immense ocean, in which lies Hawai’i, an archipelago (island chain) some 3800 km distant from the nearest continental land mass (North America) or high islands (the Marquesas). The geological history, which is quite well known, involves continued production of new volcanic material at an oceanic hotspot located in the south-east of the youngest island, Hawai’i, whose maximum age is 0.43 myo. Islands lying to the north-west are increasingly older, having been transported to their current locations (Fig. 8.5) by the north-western movement of the Pacific plate. The production of islands in this way is likened to a “conveyor belt” carrying islands away from the hotspot (which stays in the same relative position). Thus, the oldest existing above-water “high islands” (that is of greater elevation than a sand bar/atoll) are Niihau (aged 4.9 myo) and Kauia (c. 5.1 myo) positioned to the north-west. Between these two and Hawai’i lie Oahu (aged 3.7 myo), Molokai (1.9 myo), Maui and Lanai (1.3 myo), and Kahoolawe (1.0 myo). Undoubtedly there have been older islands — some estimates are that the chain originated some 80 mya — but only since about 23 mya have there been continuous high islands for colonization.
Since the islands are mid-oceanic and volcanic, they originated without any terrestrial biota, and so the present inhabitants must have descended from colonists. The great distance from source areas (other islands, the continents) implies that colonization is a rare event — and this is borne out in nearly all studies. The biota of islands is quite discordant (unbalanced) compared to that of continents. Major groups are missing, presumably by chance failure to arrive and flourish. Those that did arrive successfully and found viable populations often speciated, and may exhibit quite strange biologies with respect to their ancestors. Thus, some Hawai’ian damselflies have terrestrial larvae, in contrast to aquatic larvae elsewhere; Hawai’ian geometrid moth cater- pillars are predaceous, not phytophagous; otherwise marine midge larvae are found in freshwater torrents.
As a consequence of the rarity of founding events, most insect radiations have been identified as monophyletic, that is the complete radiation belongs to a clade derived from one founder individual or population. For some clades each species of the radiation is restricted to one island, whereas other (“widespread”) species can be found on more than one island. Fundamental to understanding the history of the colonization and subsequent diversification is a phylogeny of relationships between the species in the clade. The Hawai’ian Drosophilidae lack any widespread species (i.e. all are single island endemics) and their relationships have been studied, first with morphology and more recently with molecular techniques. Interpretation of the history of this clade is rather straight-forward; species distributions generally are congruent with the geology such that the colonists of older islands and older volcanoes (those of Oahu and Molokai) gave rise to descendants that have radiated more recently on the younger islands and younger volcanoes of Maui and Hawai’i. Similar scenarios of an older colonization with more recent radiation associated with island age are seen in the Hawai’ian Pipunculidae, damselflies, and mirids, and this probably is typical for all the diversified biota. Where estimates have been made to date the colonization and radiation, it seems few if any originated prior to the currently oldest high island (c. 5 mya), and sequential colonization seems to have been approximately contemporaneous with each newly formed island.
Aquatic insects, such as black flies (Diptera: Simuliidae) whose larvae live in running water, cannot colonize islands until persistent streams and seepages form. As islands age in geological time, greatest environmental heterogeneity with maximum aquatic habitat diversity may occur in middle-age, until senescence-induced erosion and loss of elevated areas cause extinction. In this “middle-age”, speciation may occur on a single island as specializations in different habitats, as in Hawai’ian Megalagrion damselflies. In this clade, most speciation has been associated with existing ecological larval-habitat specialists (fast-running water, seepages, plant axils, or even terrestrial habitats) colonizing and subsequently differentiating on newly formed islands as they arose from the ocean and suitable habitats became available. However, on top of this pattern there can be radiations associated with different habitats on the same island, perhaps very rapidly after initial colonization. Furthermore, examples exist showing recolonization from younger islands to older (back-founder events) that indicate substantial complexity in the evolution of some insect radiations on islands.
Sources for the original colonizers sometimes have been difficult to find because the offspring of Hawai’ian radiations often are very distinct from any prospective non-Hawai’ian relatives; however, the western or south-western Pacific is a likely source for platynine carabids, Megalagrion damselflies, and several other groups, and North America for some mirid bugs. In contrast, the evolution of the insect fauna of the Galapagos on the eastern side of the Pacific Ocean presents a rather different story to that of Hawai’i. Widespread insect species on the Galapagos predominantly are shared with Central or South America, and endemic species often have sister-group relation- ships with the nearest South American mainland, as is proposed for much of the fauna. The biting-midge (Ceratopogonidae) fauna derives apparently from many independent founding events, and similar findings come from other families of flies. Evidently, long-distance dispersal from the nearest continent outweighs in-situ speciation in generating the diversity of the Galapagos compared to Hawai’i. Nonetheless, some estimates of arrival of founders are earlier than the currently oldest islands.
Orthopteroids of the Galapagos and Hawai’i show another evolutionary feature associated with island- living — wing loss or reduction (aptery or brachyptery) in one or both sexes. Similar losses are seen in carabid beetles, with multiple losses proposed from phylogenetic analyses. Furthermore, there are extensive radiations of certain insects in the Galapagos and Hawai’i associated with underground habitats such as larva tubes and caves. Studies of the role of sexual selection — primarily female choice of mating partner (section 5.3) — suggest that this may have played an important role in species differentiation on islands, at least of crickets and fruit flies. Whether this is enhanced relative to continental situations is unclear.
All islands of the Pacific are highly impacted by the arrival and establishment of non-native species, through introductions perhaps by continued over-water colon- izations, but certainly associated with human commerce, including well-meaning biological control activities. Some accidental introductions, such as of tramp ant species (Box 1.2) and a mosquito vector of avian malaria, have affected Hawai’ian native ecosystems detrimentally across many taxa. Even parasitoids introduced to control agricultural pests have spread to native moths in remote natural habitats (section 16.5). Our unique natural laboratories for the study of evolutionary processes are being destroyed apace.
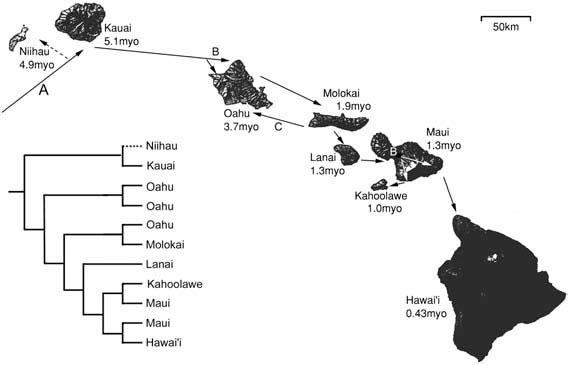
The pattern of colonization and speciation of the insects on the islands is depicted by arrows showing the sequence and direction of events: A, founding; B, diversification within an island; C, back-colonization event; myo, million years old. Broken line, extinct lineage.

