16.10. Genetic manipulation of insect pests
Cochliomyia hominivorax (Calliphoridae), the New World screw-worm fly, is a devastating pest of livestock in tropical America, laying eggs into wounds, where the larvae cause myiasis (section 15.3) by feeding in the growing suppurating wounds of the living animals, including some humans. The fly perhaps was present historically in the USA, but seasonally spread into the southern and south-western states, where substantial economic losses of stock hides and carcasses required a continuing control campaign. As the female of C. hominivorax mates only once, control can be achieved by swamping the population with infertile males, so that the first male to arrive and mate with each female is likely to be sterile and the resultant eggs inviable. The sterile male technique (also called the sterile insect technique, SIT, or the sterile insect release method, SIRM) in the Americas depends upon mass- rearing facilities, sited in Mexico, where billions of screw-worm flies are reared in artificial media of blood and casein. The larvae (Fig. 6.6h) drop to the floor of the rearing chambers, where they form a puparium. At a crucial time, after gametogenesis, sterility of the developing adult is induced by gamma-irradiation of the five-day-old puparia. This treatment sterilizes the males, and although the females cannot be separated in the pupal stage and are also released, irradiation prevents their ovipositing. The released sterile males mix with the wild population, and with each mating the fertile proportion diminishes, with eradication a theoretical possibility.
The technique has eradicated the screw-worm fly, first from Florida, then Texas and the western USA, and more recently from Mexico, from whence reinvasions of the USA once originated. The goal to create a fly-free buffer zone from Panama northwards has been attained, with progressive elimination from Central American countries and releases continuing in a permanent “sterile fly barrier” in eastern Panama. In 1990, when C. hominivorax was introduced accidentally to Libya (North Africa), the Mexican facility was able to produce enough sterile flies to prevent the establishment of this potentially devastating pest. The impressive cost/benefit ratio of screw-worm control and eradication using the sterile insect technique has induced the expenditure of substantial sums in attempts to control similar economic pests. Other examples of successful pest insect eradications involving sterile insect releases are the Mediterranean fruit fly or “medfly”, Ceratitis capitata (Tephritidae), from Mexico and northern Guatemala, the melon fly, Bactrocera cucurbitae (Tephritidae), from the Ryukyu Archipelago of Japan, and the Queensland fruit fly, Bactrocera tryoni, from Western Australia. The frequent lack of success of other ventures can be attributed to difficulties with one or more of the following:
- inability to mass culture the pest;
- lack of competitiveness of sterile males, including discrimination against captive-reared sterile males by wild females;
- genetic and phenotypic divergence of the captive population so that the sterile insects mate preferentially with each other (assortative mating);
- release of an inadequate number of males to swamp the females;
- failure of irradiated insects to mix with the wild population;
- poor dispersal of the sterile males from the release site, and rapid reinvasion of wild types.
Attempts have been made to introduce deleterious genes into pest species that can be mass cultured and released, with the intention that the detrimental genes spread through the wild population. The reasons for the failure of these attempts are likely to include those cited above for many sterile insect releases, particularly their lack of competitiveness, together with genetic drift and recombination that reduces the genetic effects.
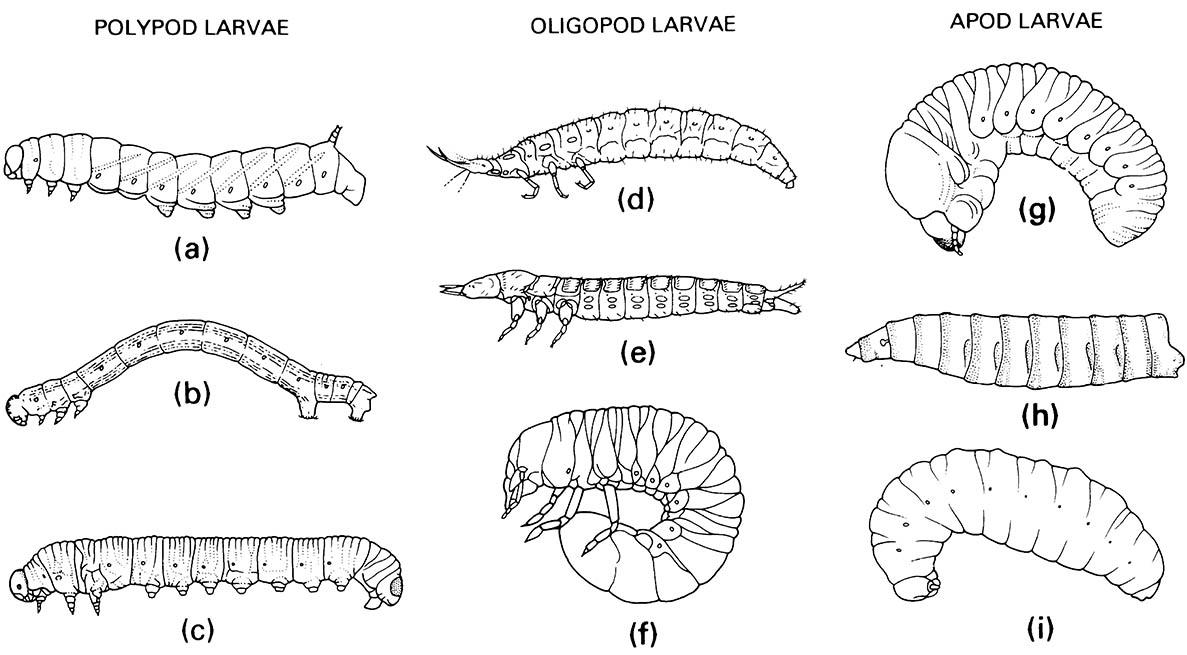
(a) Lepidoptera: Sphingidae; (b) Lepidoptera: Geometridae; (c) Hymenoptera: Diprionidae. Oligopod larvae: (d) Neuroptera: Osmylidae; (e) Coleoptera: Carabidae; (f ) Coleoptera: Scarabaeidae. Apod larvae: (g) Coleoptera: Scolytidae; (h) Diptera: Calliphoridae; (i) Hymenoptera: Vespidae. ((a, e-g) After Chu 1949; (b, c) after Borror et al. 1989; (h) after Ferrar 1987; (i) after CSIRO 1970)

