14.6. Collective defenses in gregarious and social insects
Chemically defended, aposematic insects are often clustered rather than uniformly distributed through a suitable habitat. Thus, unpalatable butterflies may live in conspicuous aggregations as larvae and as adults; the winter congregation of migratory adult monarch butterflies in California (see Plate 3.5) and Mexico is an example. Many chemically defended hemipterans aggregate on individual host plants, and some vespid wasps congregate conspicuously on the outside of their nests (as shown in the vignette of Chapter 12). Orderly clusters occur in the phytophagous larvae of sawflies (Hymenoptera: Pergidae; Fig. 14.7) and some chrysomelid beetles that form defended circles (cycloalexy). Some larvae lie within the circle and others form an outer ring with either their heads or abdomens directed outwards, depending upon which end secretes the noxious compounds. These groups often make synchronized displays of head and/or abdomen bobbing, which increase the apparency of the group.
Formation of such clusters is sometimes encouraged by the production of aggregation pheromones by early arriving individuals (section 4.3.2), or may result from the young failing to disperse after hatching from one or several egg batches. Benefits to the individual from the clustering of chemically defended insects may relate to the dynamics of predator training. However, these also may involve kin selection in subsocial insects, in which aggregations comprise relatives that benefit at the expense of an individual “sacrificed” to educate a predator.
This latter scenario for the origin and maintenance of group defense certainly seems to apply to the eusocial Hymenoptera (ants, bees, and wasps), as seen in Chapter 12. In these insects, and in the termites (Isoptera), defensive tasks are undertaken usually by morphologically modified individuals called soldiers. In all social insects, excepting the army ants, the focus for defensive action is the nest, and the major role of the soldier caste is to protect the nest and its inhabitants. Nest architecture and location is often a first line of defense, with many nests buried underground, or hidden within trees, with a few, easily defendable entrances. Exposed nests, such as those of savanna-zone termites, often have hard, impregnable walls.
Termite soldiers can be male or female, have weak sight or be blind, and have enlarged heads (sometimes exceeding the rest of the body length). Soldiers may have well-developed jaws, or be nasute, with small jaws but an elongate “nasus” or rostrum. They may protect the colony by biting, by chemical means, or, as in Cryptotermes, by phragmosis — the blocking of access to the nest with their modified heads. Amongst the most serious adversaries of termites are ants, and complex warfare takes place between the two. Termite soldiers have developed an enormous battery of chemicals, many produced in highly elaborated frontal and salivary glands. For example, in Pseudacanthotermes spiniger the salivary glands fill nine-tenths of the abdomen, and Globitermes sulphureus soldiers are filled to bursting with sticky yellow fluid used to entangle the predator — and the termite — usually fatally. This suicidal phenomenon is seen also in some Camponotus ants, which use hydrostatic pressure in the gaster to burst the abdomen and release sticky fluid from the huge salivary glands.
Some of the specialized defensive activities used by termites have developed convergently amongst ants. Thus, the soldiers of some formicines, notably the subgenus Colobopsis, and several myrmecines show phragmosis, with modifications of the head to allow the blocking of nest entrances (Fig. 14.9). Nest entrances are made by minor workers and are of such a size that the head of a single major worker (soldier) can seal it; in others such as the myrmecine Zacryptocerus, the entrances are larger, and a formation of guarding blockers may be required to act as “gatekeepers”. A further defensive strategy of these myrmecines is for the head to be covered with a crust of secreted filamentous material, such that the head is camouflaged when it blocks a nest entrance on a lichen-covered twig.
Most soldiers use their strongly developed mandibles in colony defense as a means of injuring an attacker. A novel defense in termites involves elongate mandibles that snap against one another, as we might snap our fingers. A violent movement is produced as the pent-up elastic energy is released from the tightly appressed mandibles (Fig. 14.10a). In Capritermes and Homallotermes, the mandibles are asymmetric (Fig. 14.10b) and the released pressure results in the violent movement of only the right mandible; the bent left one, which provides the elastic tension, remains immobile. These soldiers can only strike to their left! The advantage of this defense is that a powerful blow can be delivered in a confined tunnel, in which there is inadequate space to open the mandibles wide enough to obtain conventional leverage on an opponent.
Major differences between termite defenses and those of social hymenopterans are the restriction of the defensive caste to females in Hymenoptera, and the frequent use of venom injected through an ovipositor modified as a sting (Fig. 14.11). Whereas parasitic hymenopterans use this weapon to immobilize prey, in social aculeate hymenopterans it is a vital weapon in defense against predators. Many subsocial and all social hymenopterans co-operate to sting an intruder en masse, thereby escalating the effects of an individual attack and deterring even large vertebrates. The sting is injected into a predator through a lever (the furcula) acting on a fulcral arm, though fusion of the furcula to the sting base in some ants leads to a less maneuverable sting.
Venoms include a wide variety of products, many of which are polypeptides. Biogenic amines such as any or all of histamine, dopamine, adrenaline (epinephrine), and noradrenaline (norepinephrine) (and serotonin in wasps) may be accompanied by acetylcholine, and several important enzymes including phospholipases and hyaluronidases (which are highly allergenic). Wasp venoms have a number of vasopeptides — pharmacologically active kinins that induce vasodilation and relax smooth muscle in vertebrates. Non-formicine ant venoms comprise either similar materials of proteinaceous origin or a pharmacopoeia of alkaloids, or complex mixtures of both types of component. In contrast, formicine venoms are dominated by formic acid.
Venoms are produced in special glands sited on the bases of the inner valves of the ninth segment, comprising free filaments and a reservoir store, which may be simple or contain a convoluted gland (Fig. 14.11). The outlet of Dufour’s gland enters the sting base ventral to the venom duct. The products of this gland in eusocial bees and wasps are poorly known, but in ants Dufour’s gland is the site of synthesis of an astonishing array of hydrocarbons (over 40 in one species of Camponotus). These exocrine products include esters, ketones, and alcohols, and many other compounds used in communication and defense.
The sting is reduced and lost in some social hymenopterans, notably the stingless bees and formicine ants. Alternative defensive strategies have arisen in these groups; thus many stingless bees mimic stinging bees and wasps, and use their mandibles and defensive chemicals if attacked. Formicine ants retain their venom glands, which disperse formic acid through an acidophore, often directed as a spray into a wound created by the mandibles.
Other glands in social hymenopterans produce additional defensive compounds, often with communication roles, and including many volatile compounds that serve as alarm pheromones. These stimulate one or more defensive actions: they may summon more individuals to a threat, marking a predator so that the attack is targeted, or, as a last resort, they may encourage the colony to flee from the danger. Mandibular glands produce alarm pheromones in many insects and also substances that cause pain when they enter wounds caused by the mandibles. The metapleural glands in some species of ants produce compounds that defend against microorganisms in the nest through antibiotic action. Both sets of glands may produce sticky defensive substances, and a wide range of pharmacological compounds is currently under study to determine possible human benefit.
Even the best-defended insects can be parasitized by mimics, and the best of chemical defenses can be breached by a predator (Box 14.1). Although the social insects have some of the most elaborate defenses seen in the Insecta, they remain vulnerable. For example, many insects model themselves on social insects, with representatives of many orders converging morphologically on ants (Fig. 14.12), particularly with regard to the waist constriction and wing loss, and even kinked antennae. Some of the most extraordinary ant-mimicking insects are tropical African bugs of the genus Hamma (Membracidae), as exemplified by H. rectum depicted in both side and dorsal view in the vignette for this chapter.
The aposematic yellow-and-black patterns of vespid wasps and apid bees provide models for hundreds of mimics throughout the world. Not only are these communication systems of social insects parasitized, but so are their nests, which provide many parasites and inquilines with a hospitable place for their development (section 12.3).
Defense must be seen as a continuing coevolutionary process, analogous to an “arms race”, in which new defenses originate or are modified and then are selectively breached, stimulating improved defenses.
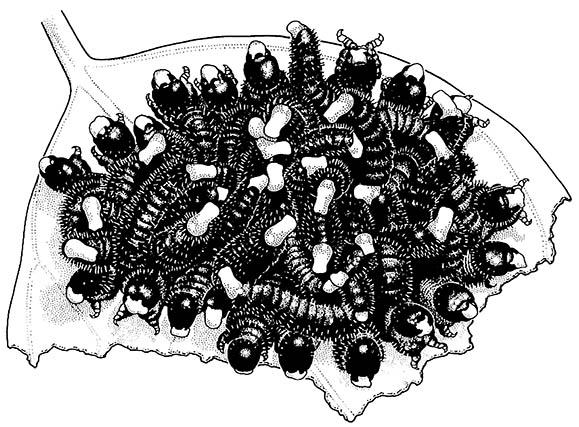
When disturbed, the larvae bend their abdomens in the air and exude droplets of sequestered eucalypt oil from their mouths.
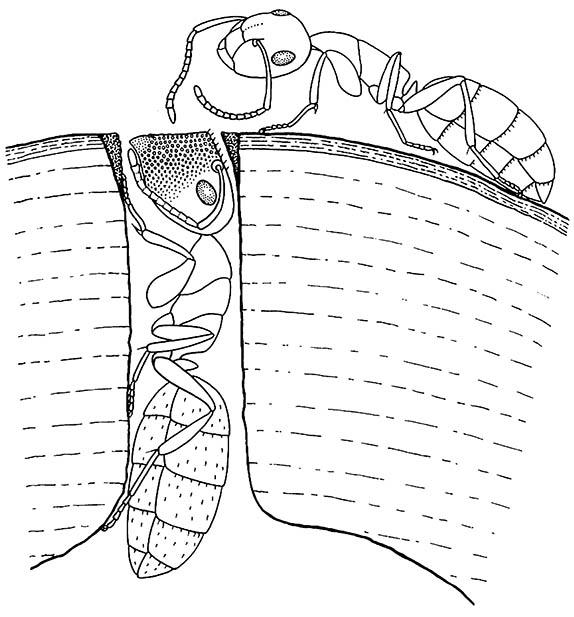
a minor worker approaching a soldier that is blocking a nest entrance with her plug-shaped head. (After Hölldobler & Wilson 1990; from Szabó-Patay 1928)
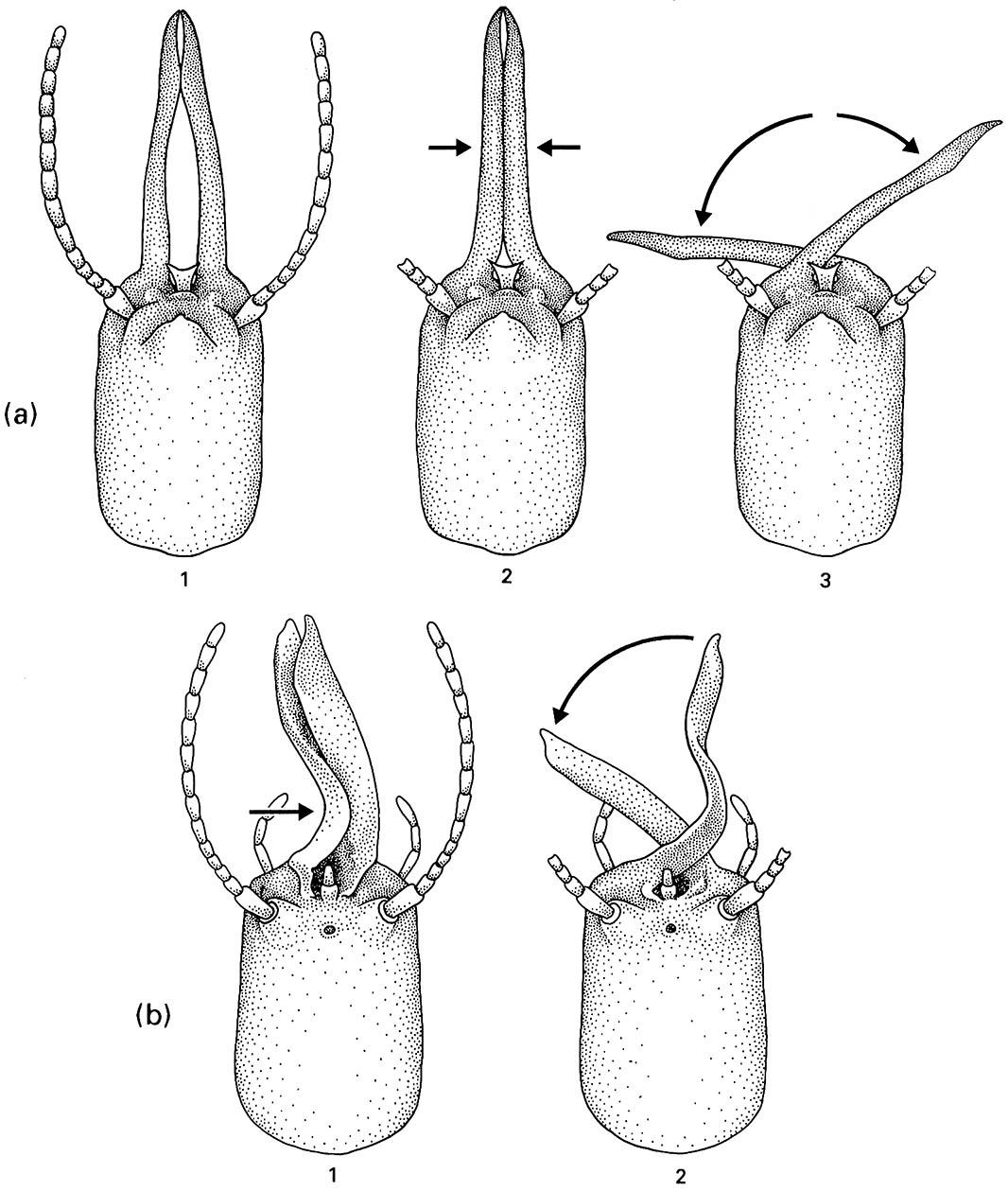
(a) Head of a symmetric snapping soldier of Termes in which the long thin mandibles are pressed hard together (1) and thus bent inwards (2) before they slide violently across one another (3). (b) Head of an asymmetric snapping soldier of Homallotermes in which force is generated in the flexible left mandible by being pushed against the right one (1) until the right mandible slips under the left one to strike a violent blow (2). (After Deligne et al. 1981)
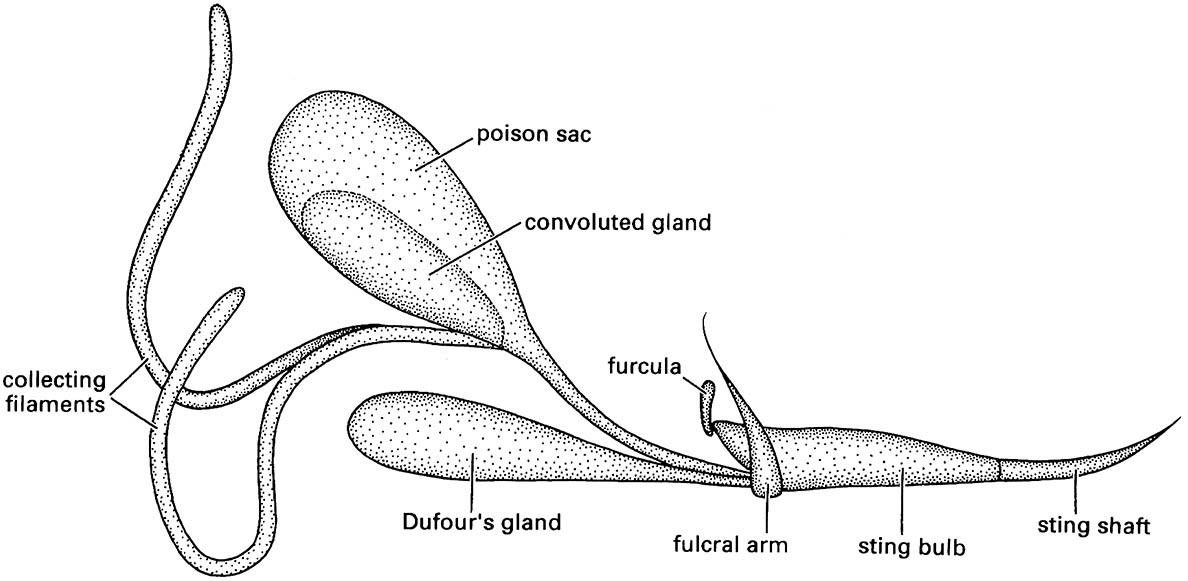
(After Hermann & Blum 1981)
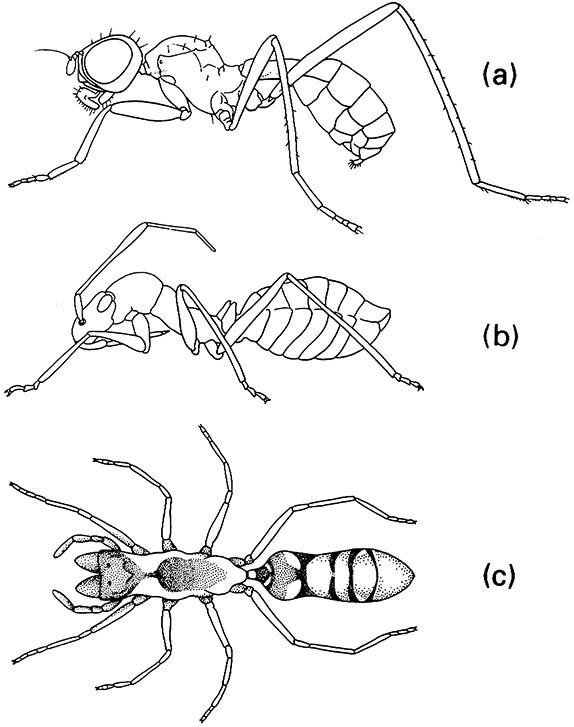
(a) a fly (Diptera: Micropezidae: Badisis); (b) a bug (Hemiptera: Miridae: Phylinae); (c) a spider (Araneae: Clubionidae: Sphecotypus). ((a) After McAlpine 1990; (b) after Atkins 1980; (c) after Oliveira 1988)

