17.2.4. Habitats, mounting, and preservation of individual orders
The following list is alphabetical (by order) and gives a summary of the usual habitats or collection methods, and recommendations for mounting and preserving each kind of insect or other hexapod. Insects that are to be pinned and stored dry can be killed either in a freezer or in a killing bottle (section 17.2.1); the list also specifies those insects that should be preserved in ethanol or fixed in another fluid prior to preservation (section 17.2.2). Generally, 75–80% ethanol is suggested for liquid storage, but the preferred strength often differs between collectors and depends on the kind of insect. For detailed instructions on how to collect and preserve different insects, refer to the publications in the further reading list at the end of this chapter.
Archaeognatha (bristletails)
These occur in leaf litter, under bark, or similar situations. Collect into and preserve in 80% ethanol.
Blattodea (roaches, cockroaches)
These are ubiquitous, found in sites ranging from peridomestic to native vegetation, including caves and burrows; they are predominantly nocturnal. Eviscerate large specimens, and pin through the center of the metanotum — wings of the left side may be spread. They may also be preserved in 80% ethanol.
Coleoptera (beetles)
Beetles are found in all habitats. Pin adults and store dry; pin through the right elytron near its front so that the pin emerges between the mid and hind legs (Figs. 17.2a, 17.3, & 17.6a). Mount smaller specimens on card points with the apex of the point bent down slightly (Fig. 17.5b) and contacting the posterior lateral thorax between the mid and hind pair of legs. Immature stages are preserved in fluid (stored in 85–90% ethanol, preferably after fixation in KAA or Carnoy’s fluid).
Collembola (springtails)
These are found in soil, litter, and at water surfaces (fresh and intertidal). Collect into 95–100% ethanol and preserve on microscope slides.
Dermaptera (earwigs)
Favored locations include litter, under bark or logs, dead vegetation (including along the shoreline), and in caves; exceptionally they are ectoparasitic on bats. Pin through the right elytron and with the left wings spread. Collect a representative sample of immature stages into Pampel’s fluid and then 75% ethanol.
Diplura (diplurans)
These occur in damp soil under rocks or logs. Collect into 75% ethanol; preserve in 75% ethanol or slide mount.
Diptera (flies)
Flies are found in all habitats. Pin adult specimens and store dry, or preserve in 75% ethanol; pin most adults to right of center of the mesothorax (Fig. 17.2c); stage or cube mount smaller specimens (Fig. 17.4c,d) (card pointing not recommended). Collect immature stages into Pampel’s fluid (larger) or 75% ethanol (smaller). Slide mount smaller adults and the larvae of some families.
Embiidina (or Embioptera; embiids or webspinners) Typical locations for the silken galleries of webspinners are in or on bark, lichens, rocks, or wood. Preserve and store in 75% ethanol or slide mount; winged adults can be pinned through the center of the thorax with wings spread.
Ephemeroptera (mayflies)
Adults occur beside water. Preserve in 75% ethanol (preferably after fixing in Carnoy’s fluid or FAA) or pin through the center of the thorax with the wings spread. Immature stages are aquatic. Collect these into and preserve in 75% ethanol or first fix in Carnoy’s fluid or FAA, or store dissected on slides or in microvials.
Grylloblattodea (rock crawlers or ice crawlers)
These can be collected on or under rocks, or on snow or ice. Preserve specimens in 75% ethanol (preferably after fixing in Pampel’s fluid), or slide mount.
Hemiptera
The Cicadomorpha (cicadas, leafhoppers, spittle bugs), Fulgoromorpha (planthoppers), and Heteroptera (true bugs) are associated with their host plants or are predaceous and free-living; aquatic forms also have these habits. Preserve the adults dry; pin through the scutellum or thorax to the right of center (Fig. 17.2h); spread the wings of cicadas and fulgoroids, point or stage smaller specimens (Fig. 17.4a). Preserve nymphs in 80% ethanol.
The Aphidoidea (aphids) and Coccoidea (scale insects, mealybugs) are found associated with their host plants, including leaves, stems, roots, and galls. Store nymphs and adults in lactic-alcohol or 80% ethanol, or dry on a plant part; slide mount to identify.
Aleyrodoidea (whiteflies) are associated with their host plants. The sessile final-instar nymph (“puparium”) or its exuviae (“pupal case”) are of taxonomic importance. Preserve all stages in 80–95% ethanol; slide mount puparia or pupal cases.
The Psylloidea (psyllids, lerps) are associated with host plants; rear nymphs to obtain adults. Preserve nymphs in 80% ethanol, dry mount galls or lerps (if present). Preserve adults in 80% ethanol or dry mount on points; slide mount dissected parts.
Hymenoptera (ants, bees, sawflies, and wasps)
Hymenoptera are ubiquitous, and many are parasitic, in which case the host association should be retained. Collect bees, sawflies, and wasps into 80% ethanol or pin and store dry — pin larger adults to the right of center of the mesothorax (Fig. 17.2e) (sometimes with the pin angled to miss the base of the fore legs); point mount smaller adults (Fig. 17.5a); slide mount if very small. Immature stages should be preserved in 80% ethanol, often after fixing in Carnoy’s fluid or KAA. Ants require stronger ethanol (90–95%); point a series of ants from each nest, with each ant glued on to the upper apex of the point between the mid and hind pairs of legs (Fig. 17.5c); two or three ants from a single nest can be mounted on separate points on a single macropin.
Isoptera (termites or “white ants”)
Collect termites from colonies in mounds, on live or dead trees, or below ground. Preserve all castes available in 80% ethanol.
Lepidoptera (butterflies and moths)
Lepidoptera are ubiquitous. Collect by netting and (especially moths) at a light. Pin vertically through the thorax and spread the wings so that the hind margins of the fore wings are at right angles to the body (Figs. 17.2d & 17.6b). Microlepidopterans are best micropinned (Fig. 17.4b) immediately after death. Immature stages are killed in KAA or boiling water, and transferred to 85–95% ethanol.
Mantodea (mantids)
These are generally found on vegetation, sometimes attracted to light at night. Rear the nymphs to obtain adults. Eviscerate larger specimens. Pin between the wing bases and set the wings on the left side (Fig. 17.6b).
Mantophasmatodea (heel walkers)
These are found on mountains in Namibia by day, and also at lower elevations in South Africa at night. Pin mid-thorax, or transfer into 80–90% ethanol.
Mecoptera (scorpionflies or hangingflies)
Mecoptera often occur in damp habitats, near streams or wet meadows. Pin adults to the right of center of the thorax with the wings spread. Alternatively, all stages may be fixed in KAA, FAA, or 80% ethanol and preserved in 80% ethanol.
Megaloptera (alderflies or dobsonflies)
These are usually found in damp habitats, often near streams and lakes. Pin adults to the right of center of the thorax with the wings spread. Alternatively, all stages can be fixed in FAA or 80% ethanol and preserved in ethanol.
Neuroptera (lacewings and antlions)
Neuroptera are ubiquitous, associated with vegetation, sometimes in damp places. Pin adults to the right of center of the thorax with the wings spread (Fig. 17.2f ) and the body supported. Alternatively, preserve in 80% ethanol. Immature stages are fixed in KAA, Carnoy’s fluid, or 80% ethanol, and preserved in ethanol.
Odonata (damselflies and dragonflies)
Although generally found near water, adult odonates may disperse and migrate; the nymphs are aquatic. If possible keep the adult alive and starve for 1–2 days before killing (this helps to preserve body colors after death). Pin through the mid-line of the thorax between the wings, with the pin emerging between the first and second pair of legs (Fig. 17.2g); set the wings with the front margins of the hind wings at right angles to the body (a good setting method is to place the newly pinned odonate upside down with the head of the pin pushed into a foam drying board). Preserve immature stages in 80% ethanol; the exuviae should be placed on a card associated with adult.
Orthoptera (grasshoppers, locusts, crickets, katydids)
Orthoptera are found in most terrestrial habitats. Remove the gut from all but the smallest specimens, and pin vertically through the right posterior quarter of the prothorax, spreading the left wings (Fig. 17.2b). Nymphs and soft-bodied adults should be fixed in Pampel’s fluid then preserved in 75% ethanol.
Phasmatodea (phasmatids, phasmids, stick-insects or walking sticks)
These are found on vegetation, usually nocturnally (sometimes attracted to light). Rear the nymphs to obtain adults, and remove the gut from all but the smallest specimens. Pin through the base of the mesothorax with the pin emerging between bases of the mesothoracic legs, spread the left wings, and fold the antennae back along the body.
Phthiraptera (lice)
Lice can be seen on their live hosts by inspecting the plumage or pelt, and can be removed using an ethanol-soaked paintbrush. Lice depart recently dead hosts as the temperature drops — and can be picked from a dark cloth background. Ectoparasites also can be removed from a live host by keeping the host’s head free from a bag enclosing the rest of the body and containing chloroform to kill the parasites, which can be shaken free, and leaving the host unharmed. Legislation concerning the handling of hosts and of chloroform render this a specialized technique. Lice are preserved in 80% ethanol and slide mounted.
Plecoptera (stoneflies)
Adult plecopterans are restricted to the proximity of aquatic habitats. Net or pick from the substrate, infrequently attracted to light. Nymphs are aquatic, being found especially under stones. Pin adults through the center of the thorax with the wings spread, or preserve in 80% ethanol. Immature stages are preserved in 80% ethanol, or dissected on slides or in microvials.
Protura (proturans)
Proturans are most easily collected by extracting from litter using a Tullgren funnel. Collect into and preserve in 80% ethanol, or slide mount.
Psocoptera (psocids; barklice and booklice)
Psocids occur on foliage, bark, and damp wooden surfaces, sometimes in stored products. Collect with an aspirator or ethanol-laden paintbrush into 80% ethanol; slide mount small specimens.
Raphidioptera (snakeflies)
These are typically found in damp habitats, often near streams and lakes. Pin adults or fix in FAA or 80% ethanol; immature stages are preserved in 80% ethanol.
Siphonaptera (fleas)
Fleas can be removed from a host bird or mammal by methods similar to those outlined above for Phthiraptera. If free-living in a nest, use fine forceps or an alcohol-laden brush. Collect adults and larvae into 75–80% ethanol; preserve in ethanol or by slide mounting.
Strepsiptera (strepsipterans)
Adult males are winged, whereas females and immature stages are endoparasitic, especially in leafhoppers and planthoppers (Hemiptera) and Hymenoptera. Preserve in 80% ethanol or by slide mounting.
Thysanoptera (thrips)
Thrips are common in flowers, fungi, leaf litter, and some galls. Collect adults and nymphs into AGA or 60–90% ethanol and preserve by slide mounting.
Trichoptera (caddisflies)
Adult caddisflies are found beside water and attracted to light, and immature stages are aquatic in all waters. Pin adults through the right of center of the mesonotum with the wings spread, or preserve in 80% ethanol. Immature stages are fixed in FAA or 75% ethanol, and preserved into 80% ethanol. Micro-caddisflies and dissected nymphs are preserved by slide mounting.
Zoraptera (zorapterans)
These occur in rotten wood and under bark, with some found in termite nests. Preserve in 75% ethanol or slide mount.
Zygentoma (or Thysanura; silverfish)
Silverfish are peridomestic, and also occur in leaf litter, under bark, in caves, and with termites and ants. They are often nocturnal, and elusive to normal handling. Collect by stunning with ethanol, or using Tullgren funnels; preserve with 80% ethanol.
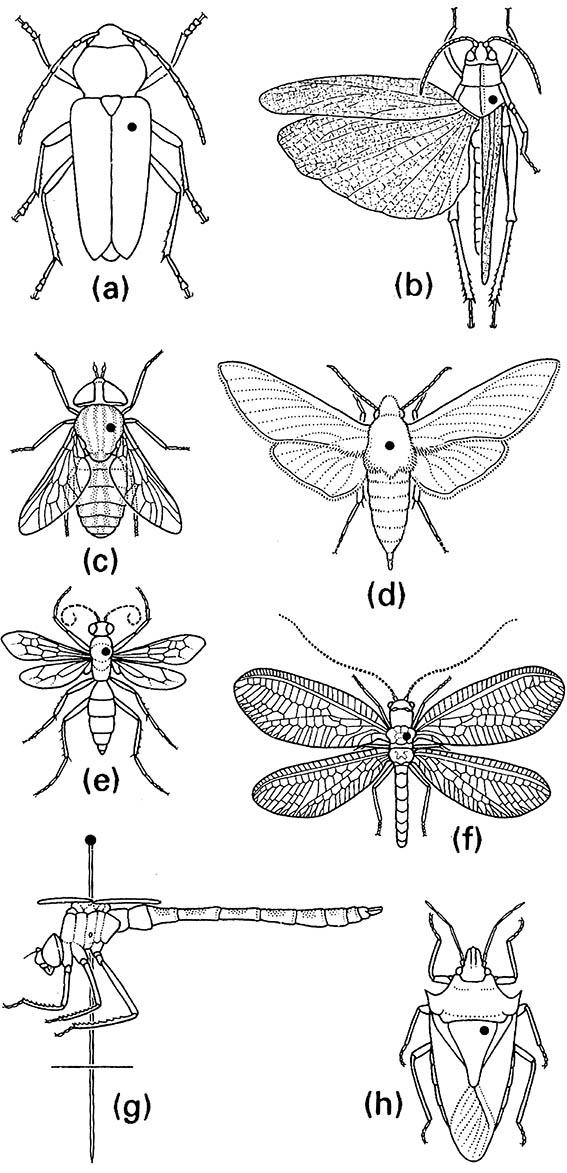
(a) larger beetles (Coleoptera); (b) grasshoppers, katydids, and crickets (Orthoptera); (c) larger flies (Diptera); (d) moths and butterflies (Lepidoptera); (e) wasps and sawflies (Hymenoptera); (f ) lacewings (Neuroptera); (g) dragonflies and damselflies (Odonata), lateral view; (h) bugs, cicadas, and leaf- and planthoppers (Hemiptera: Heteroptera, Cicadomorpha, and Fulgoromorpha).
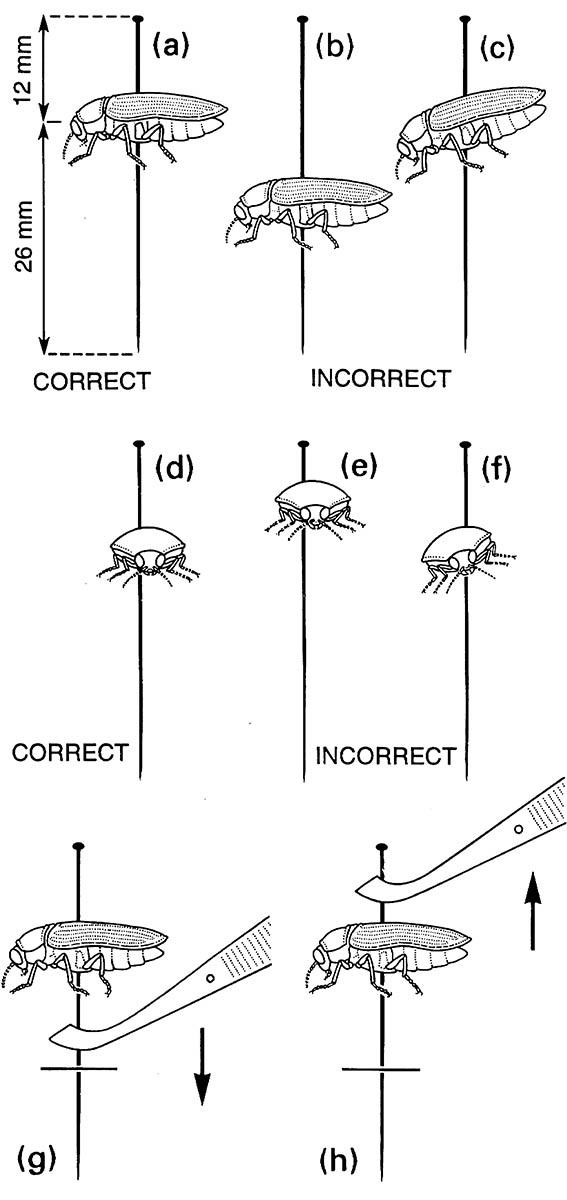
(a) insect in lateral view, correctly positioned; (b) too low on pin; (c) tilted on long axis, instead of horizontal; (d) insect in front view, correctly positioned; (e) too high on pin; (f) body tilted laterally and pin position incorrect. Handling insect specimens with entomological forceps: (g) placing specimen mount into foam or cork; (h) removing mount from foam or cork. ((g, h) After Upton 1991)
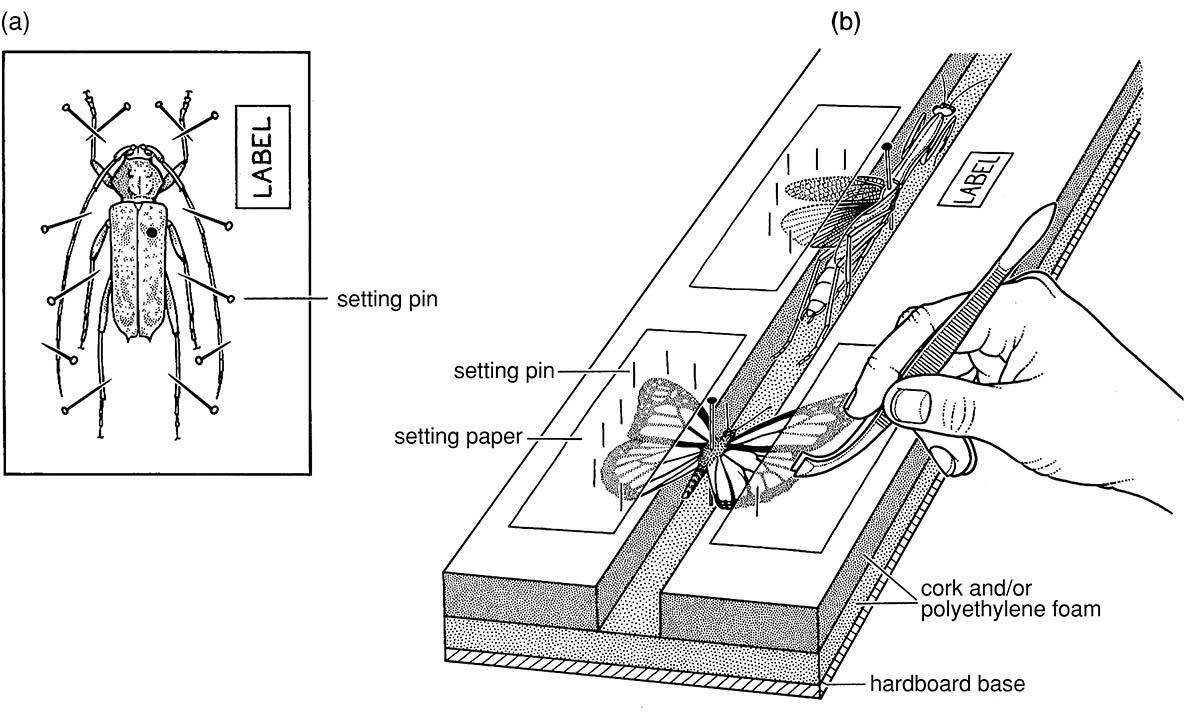
(a) a beetle pinned to a foam sheet showing the spread antennae and legs held with pins; (b) setting board with mantid and butterfly showing spread wings held in place by pinned setting paper. ((b) After Upton 1991)
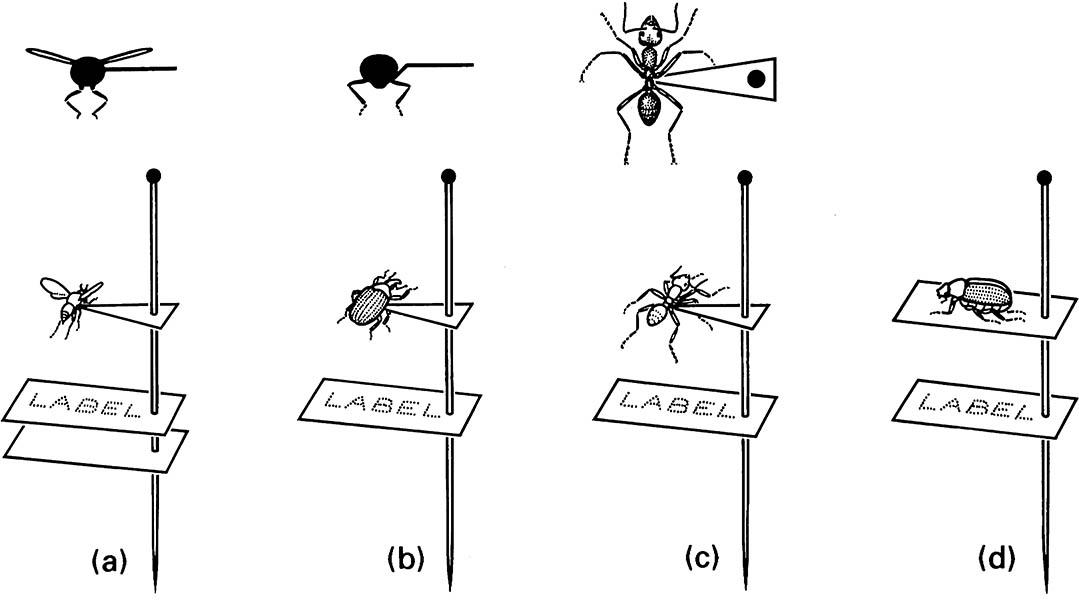
(a) a small wasp; (b) a weevil; (c) an ant. Carding: (d) a beetle glued to a card mount. (After Upton 1991)
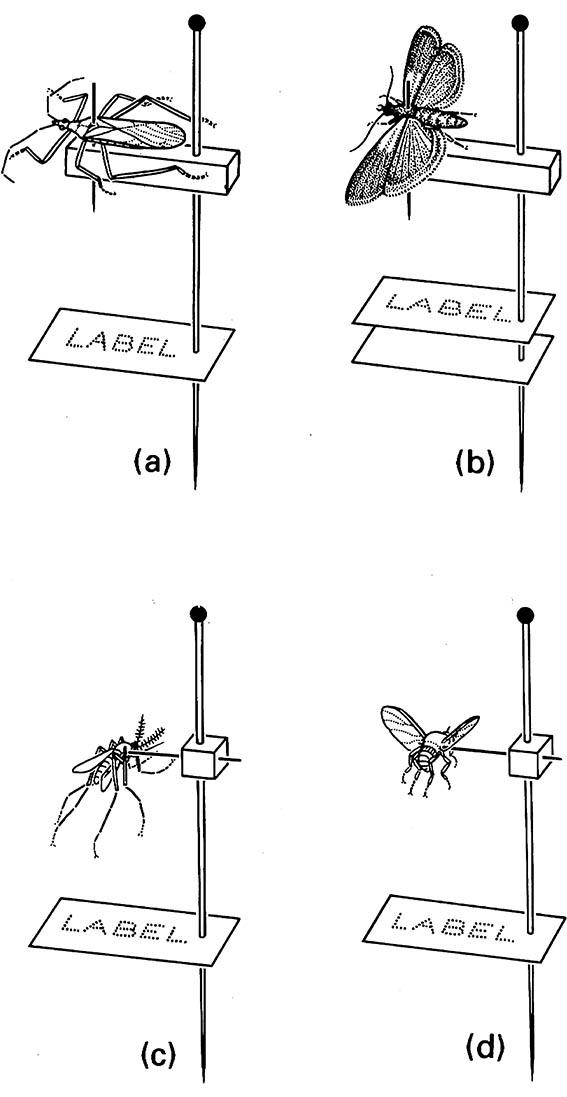
(a) a small bug (Hemiptera) on a stage mount, with position of pin in thorax as shown in Fig. 17.2h; (b) moth (Lepidoptera) on a stage mount, with position of pin in thorax; (c) mosquito (Diptera: Culicidae) on a cube mount, with thorax impaled laterally; (d) black fly (Diptera: Simuliidae) on a cube mount, with thorax impaled laterally. (After Upton 1991)

