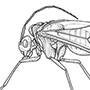
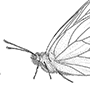
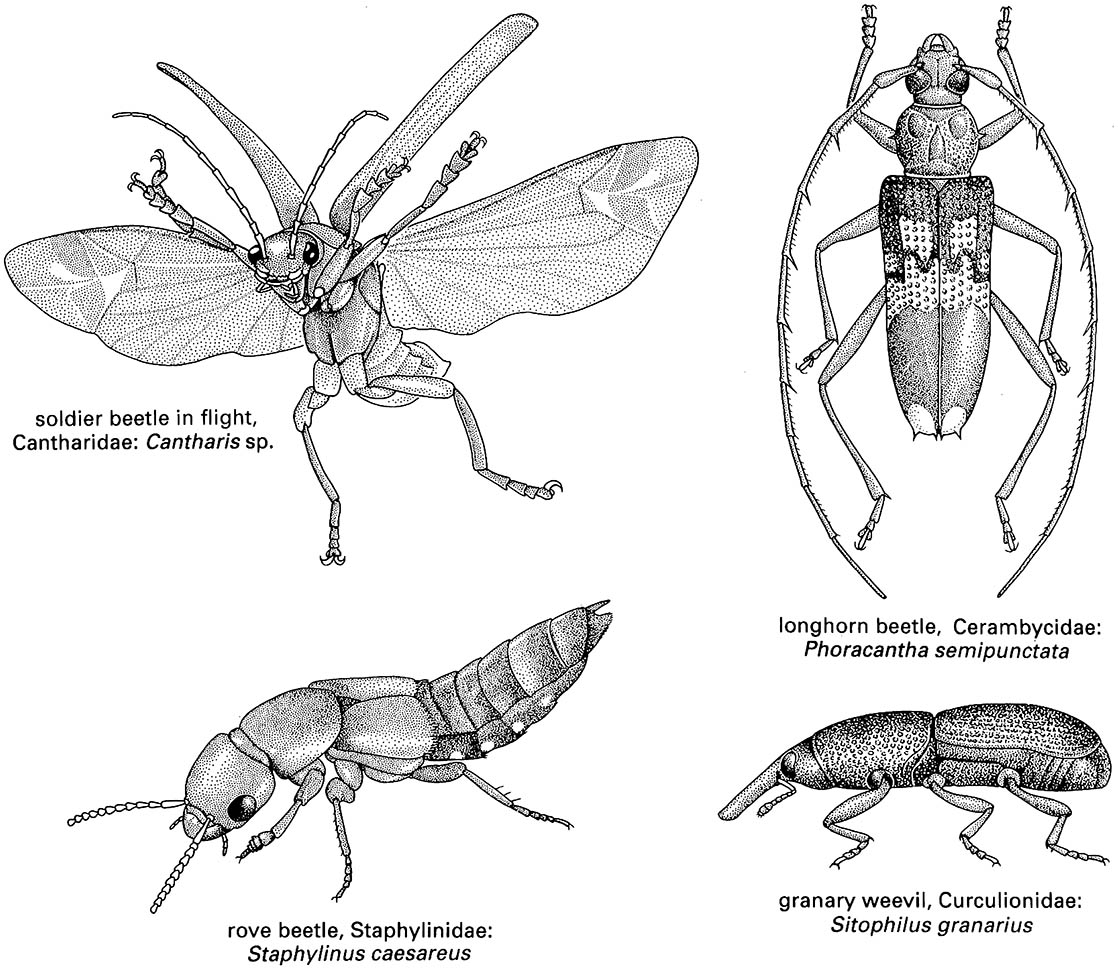
Box 11.10. Coleoptera (beetles)
The Coleoptera is probably the largest order of insects, with some 350,000 described species in four suborders (Archostemata, Myxophaga, Adephaga, and the speciose Polyphaga). Although the family-level classification is unstable, some 500 families and subfamilies are recognized. Adult beetles range from small to very large, but are usually heavily sclerotized, sometimes even armored, and often compact. Development is holometabolous. The mouthparts are mandibulate, and compound eyes range from well developed (sometimes even meeting medially) to absent; ocelli are usually absent. The antennae comprise 11 or frequently fewer segments (exceptionally with 20 segments in male Rhipiceridae). The prothorax is distinct, large, and extends laterally beyond the coxae; the mesothorax is small (at least dorsally), and fused to the metathorax to form the wing-bearing pterothorax. The fore wings are modified as sclerotized, rigid elytra (Fig. 2.22d & Plate 1.2, facing here), whose movement may assist in lift or may be restricted to opening and closing before and after flight; the elytra cover the hind wings and abdominal spiracles allowing control of water loss.
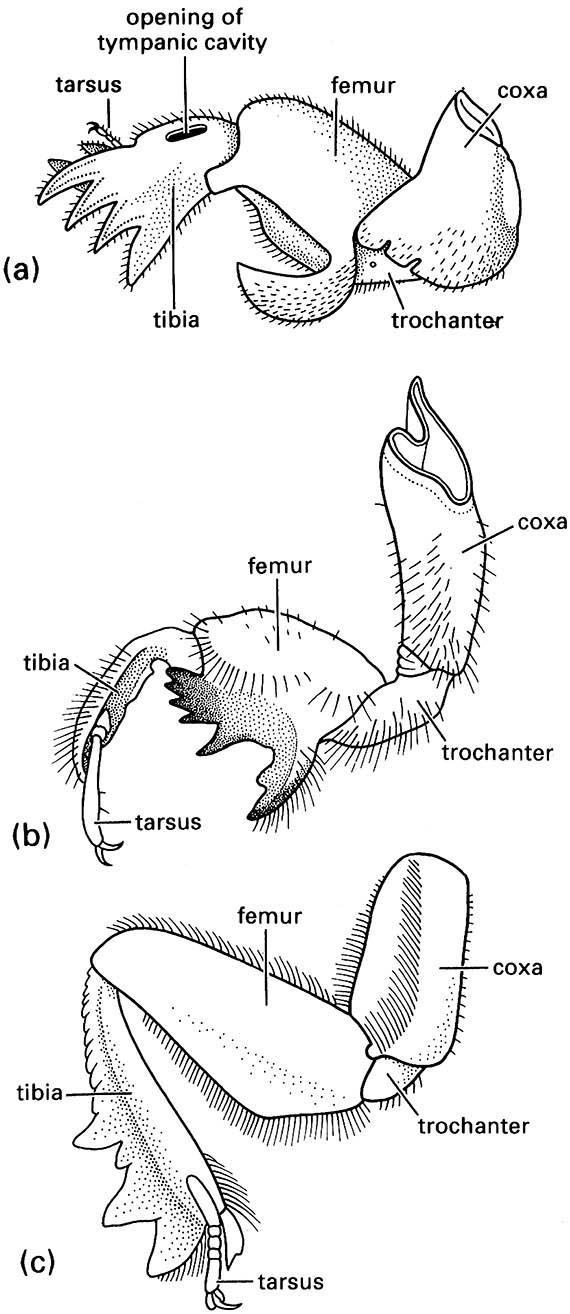
The hind wings are longer than the elytra when extended for flight (as illustrated on the upper left for a soldier beetle, Cantharis sp. (Cantharidae), after Brackenbury 1990), and have variably reduced venation, much of which is associated with complex pleating to allow the wings to be folded longitudinally and transversely beneath the elytra even if the latter are reduced in size, as in rove beetles (Staphylinidae) such as Staphylinus caesareus (illustrated on the lower left, after Stanek 1969). The legs are very variably developed, with coxae that are sometimes large and mobile; the tarsi are primitively five- segmented, although often with a reduced number of segments, and bear variously shaped claws and adhesive structures (Fig. 10.3). Sometimes the legs are fossorial (Fig. 9.2c) for digging in soil or wood, or modified for swimming (Figs. 10.3 & 10.8) or jumping. The abdomen is primitively nine-segmented in females, and 10-segmented in males, with at least one terminal segment retracted; the sterna are usually strongly sclerotized, often more so than the terga. Females have a substitutional ovipositor, whereas the male external genitalia are primitively trilobed (Fig. 2.24b). Cerci are absent.
Larvae exhibit a wide range of morphologies, but most can be recognized by the sclerotized head capsule with opposable mandibles and their usually five-segmented thoracic legs, and can be distinguished from similar lepidopteran larvae by the lack of ventral abdominal crochet-bearing prolegs and lack of a median labial silk gland. Similar symphytan wasp larvae have prolegs on abdominal segments 2–7. Beetle larvae vary in body shape and leg structure; some are apodous (lacking any thoracic legs; Fig. 6.6g), whereas legged larvae may be campodeiform (prognathous with long thoracic legs; Fig. 6.6e), eruciform (grub-like with short legs), or scarabaeiform (grub-like but long-legged; Fig. 6.6f ). Pupation is often in a specially constructed cell or chamber (Fig. 9.1), rarely in a cocoon spun from silk from Malpighian tubules, or exposed as in coccinellids (Fig. 6.7j).
Beetles occupy virtually every conceivable habitat, including freshwater (Box 10.6), a few marine and intertidal habitats, and, above all, every vegetational microhabitat from external foliage (Fig. 11.1), flowers, buds, stems, bark, and roots, to internal sites such as in galls in any living plant tissue or in any kind of dead material in all its various states of decomposition. Saprophagy and fungivory are fairly common, and dung and carrion are exploited (sections 9.3 & 9.4, respectively). Few beetles are parasitic but carnivory is frequent, occurring in nearly all Adephaga and many Polyphaga, including Lampyridae (fireflies) and many Coccinellidae (ladybird beetles; vignette to Chapter 16, Fig. 5.9). Herbivorous Chrysomelidae and Curculionidae are widely intro- duced as biological control agents of weedy plants, and Coccinellidae have been used as biological control agents for aphid and coccoid pests of plants (Box 16.2). Some beetles are significant pests of roots in pastures and crops (especially larval Scarabaeidae), of timber (especially Cerambycidae such as Phoracantha semipunctata, illustrated on the upper right, after Duffy 1963), and of stored products (such as the granary weevil, Sitophilus granarius (Curculionidae), illustrated on the lower right). These last beetles tend to be adapted to dry conditions and thrive on stored grains, cereals, pulses, and dried animal material such as skins and leather.
Phylogenetic relationships are considered in section 7.4.2 and depicted in Fig. 7.2.
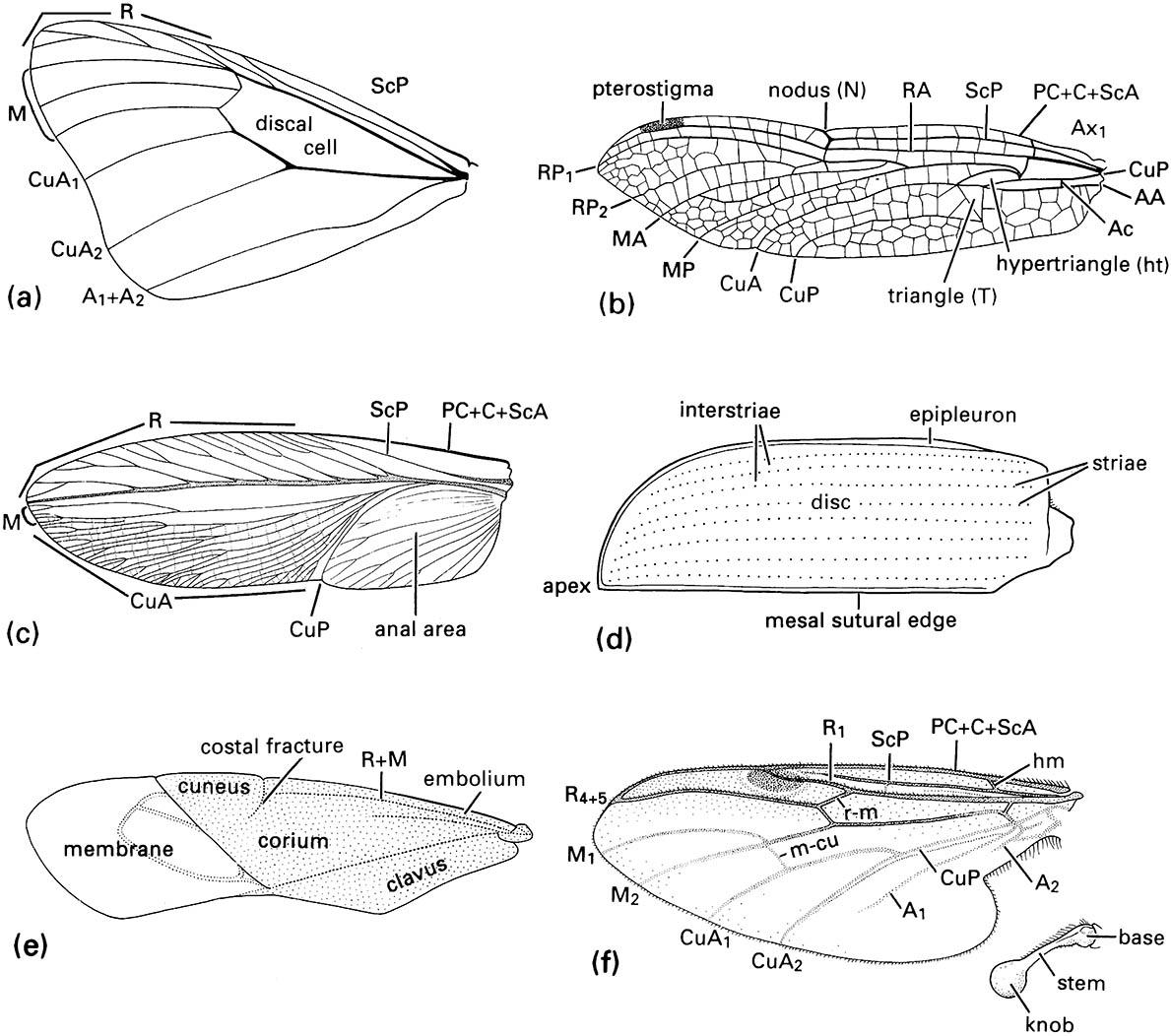
(a) fore wing of a butterfly of Danaus (Lepidoptera: Nymphalidae); (b) fore wing of a dragonfly of Urothemis (Odonata: Anisoptera: Libellulidae); (c) fore wing or tegmen of a cockroach of Periplaneta (Blattodea: Blattidae); (d) fore wing or elytron of a beetle of Anomala (Coleoptera: Scarabaeidae); (e) fore wing or hemelytron of a mirid bug (Hemiptera: Heteroptera: Miridae) showing three wing areas — the membrane, corium, and clavus; (f ) fore wing and haltere of a fly of Bibio (Diptera: Bibionidae) (after J.W.H. Trueman, unpublished. ((a-d) After Youdeowei 1977; (f) after McAlpine 1981)
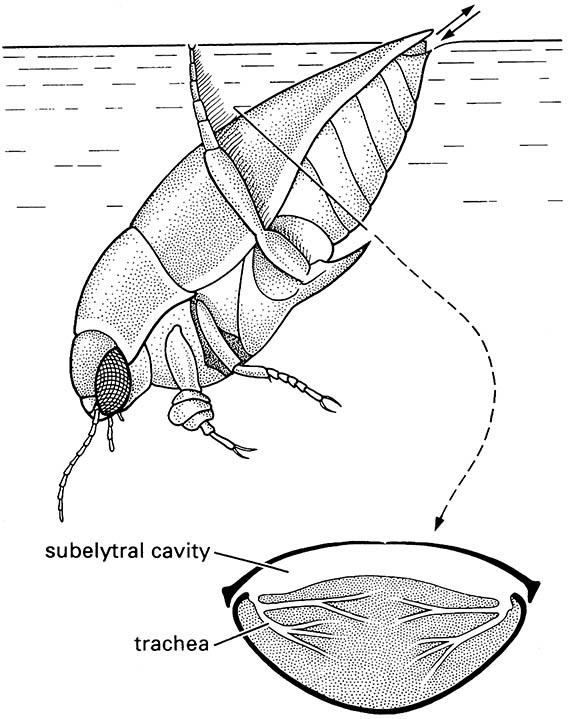
Below is a transverse section of the beetle’s abdomen showing the large air store below the elytra and the tracheae opening into this air space. Note: the tarsi of the fore legs are dilated to form adhesive pads that are used to hold the female during copulation. (Inset after Wigglesworth 1964)
(a) a mole cricket of Gryllotalpa (Orthoptera: Gryllotalpidae); (b) a nymphal periodical cicada of Magicicada (Hemiptera: Cicadidae); and (c) a scarab beetle of Canthon (Coleoptera: Scarabaeidae). ((a) After Frost 1959; (b) after Snodgrass 1967; (c) after Richards & Davies 1977)
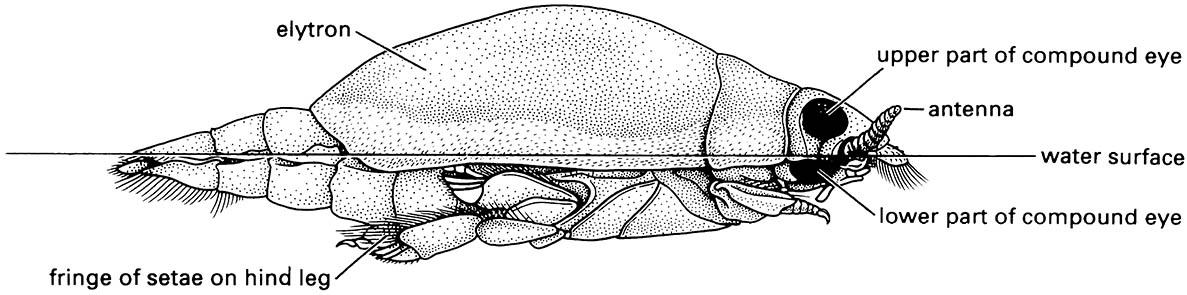
Note: the divided compound eye allows the beetle to see both above and below water simultaneously; hydrofuge hairs on the margin of the elytra repel water. (After White et al. 1984)
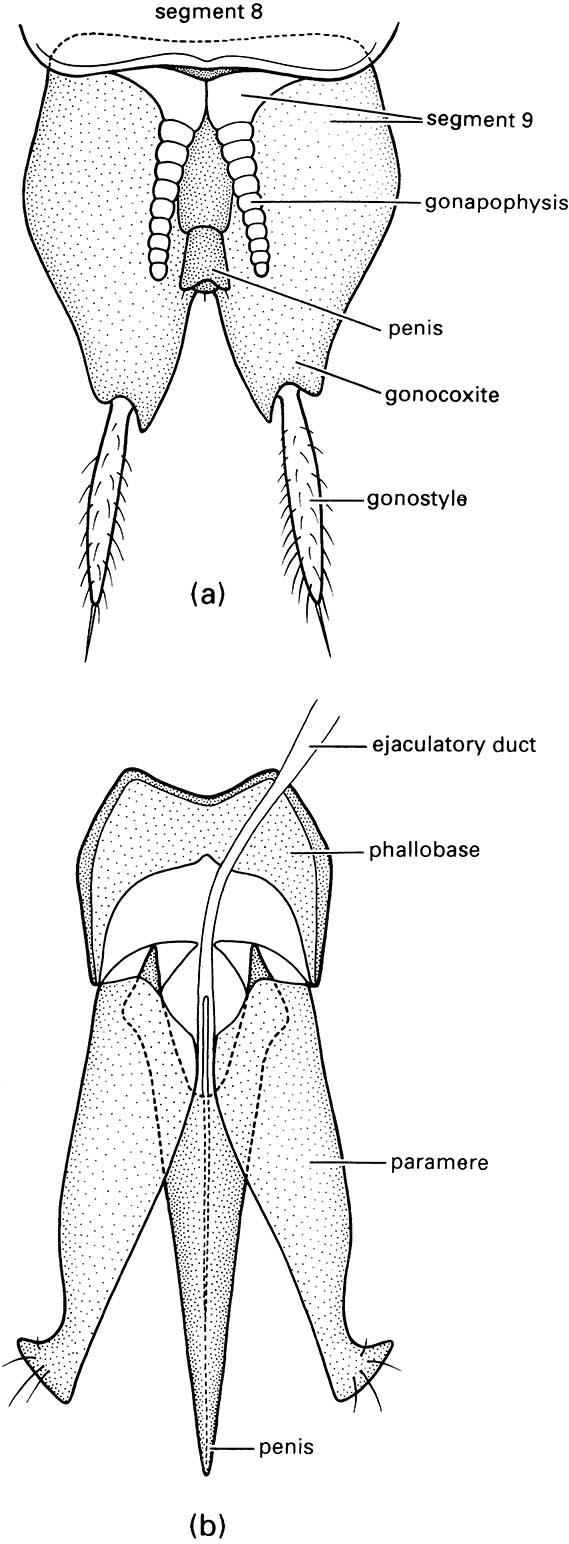
(a) Abdominal segment 9 of the bristletail Machilis variabilis (Archaeognatha: Machilidae). (b) Aedeagus of a click beetle (Coleoptera: Elateridae). ((a) After Snodgrass 1957)
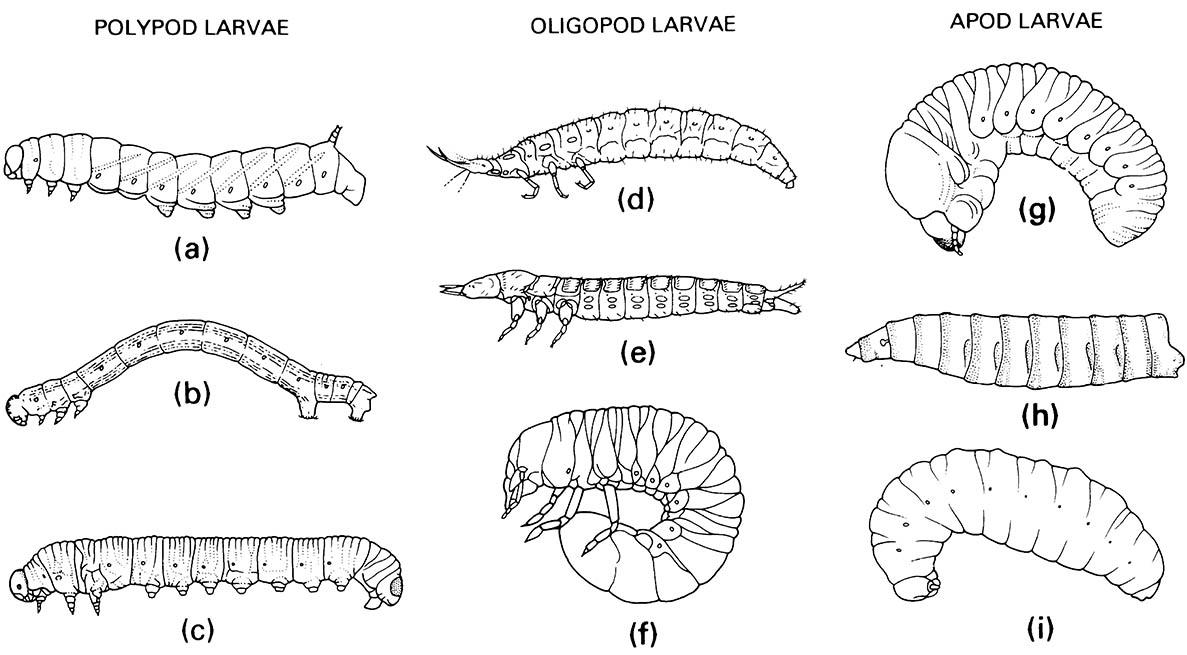
(a) Lepidoptera: Sphingidae; (b) Lepidoptera: Geometridae; (c) Hymenoptera: Diprionidae. Oligopod larvae: (d) Neuroptera: Osmylidae; (e) Coleoptera: Carabidae; (f ) Coleoptera: Scarabaeidae. Apod larvae: (g) Coleoptera: Scolytidae; (h) Diptera: Calliphoridae; (i) Hymenoptera: Vespidae. ((a, e-g) After Chu 1949; (b, c) after Borror et al. 1989; (h) after Ferrar 1987; (i) after CSIRO 1970)
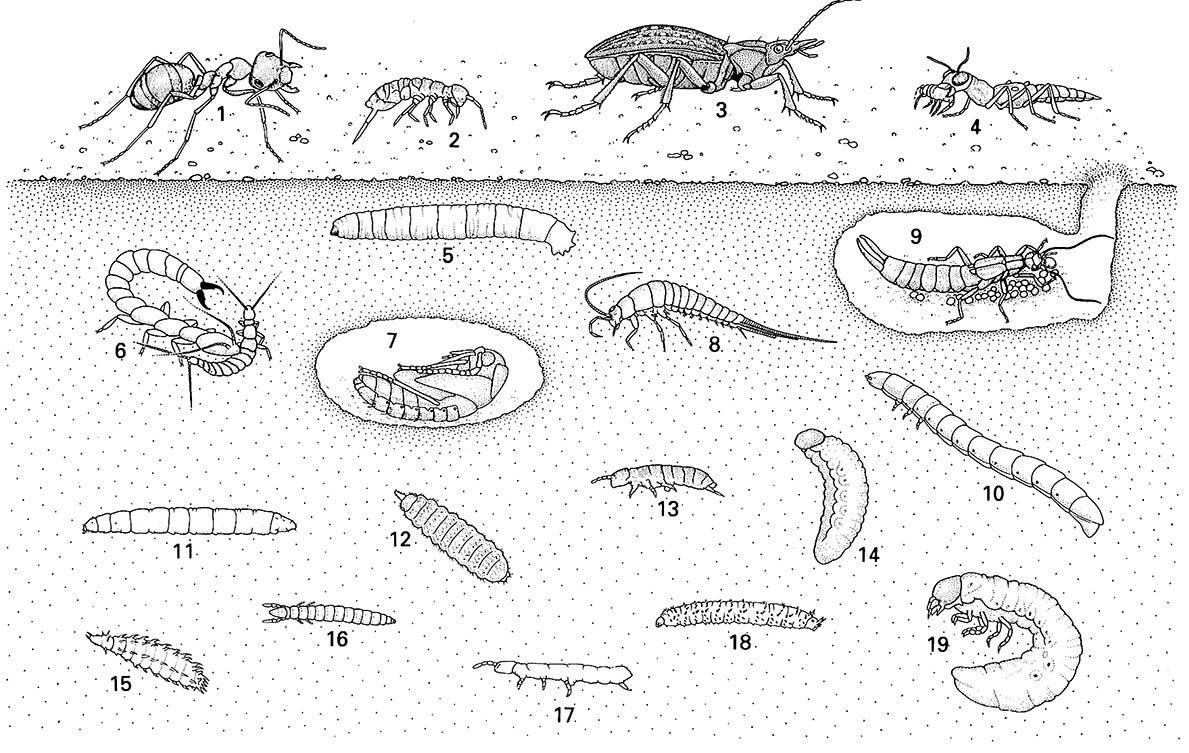
Note that organisms living on the soil surface and in litter have longer legs than those found deeper in the ground. Organisms occurring deep in th e soil usually are legless or have reduced legs; they are unpigmented and often blind. The organisms depicted are: (1) worker of a woo d ant (Hymenoptera: Formicidae); (2) springtail (Collembola: Isotomidae); (3) ground beetle (Coleoptera: Carabidae); (4) rove beetle (Coleoptera: Staphylinidae) eating a springtail; (5) larva of a crane fly (Diptera: Tipulidae); (6) japygid dipluran (Dip lura: Japygidae) attacking a smaller campodeid dipluran; (7) pupa of a ground beetle (Coleoptera: Carabidae); (8) bristletail (Archaeognatha: Machilidae); (9) female earwig (Dermaptera: Labiduridae) tending her eggs; (10) wireworm, larva of a tenebrionid beetle (Coleoptera: Tenebrionidae); (11) larva of a robber fly (Diptera: Asilidae); (12) larva of a soldier fly (Dipt era: Stratiomyidae); (13) springtail (Collembola: Isotomidae); (14) larva of a weevil (Coleoptera: Curculionidae); (15) larva of a m uscid fly (Diptera: Muscidae); (16) proturan (Protura: Sinentomidae); (17) springtail (Collembola: Isotomidae); (18) larva of a March fly (Diptera: Bibionidae); (19) larva of a scarab beetle (Coleoptera: Scarabaeidae). (Individual organisms after various sources, especially Eisenbeis & Wichard 1987)
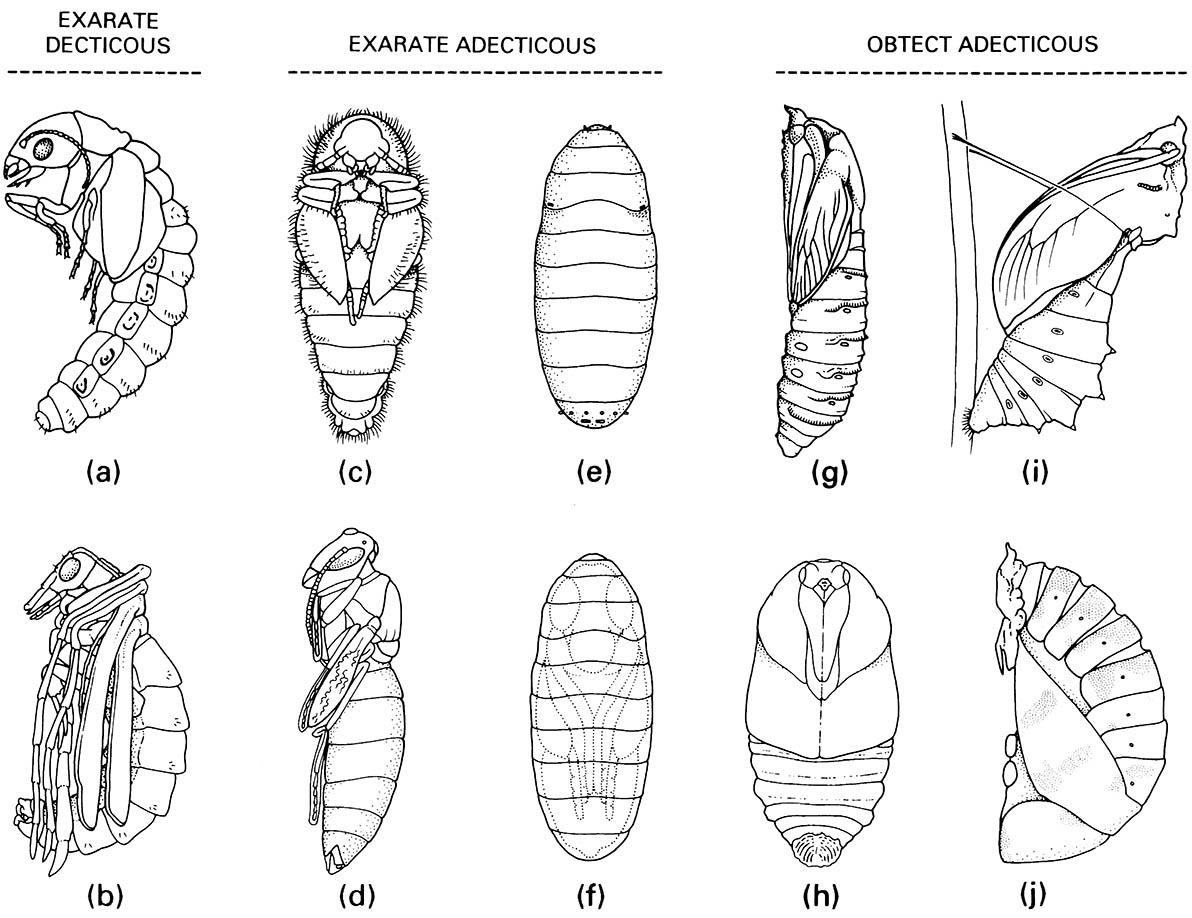
Exarate decticous pupae: (a) Megaloptera: Sialidae; (b) Mecoptera: Bittacidae. Exarate adecticous pupae: (c) Coleoptera: Dermestidae; (d) Hymenoptera: Vespidae; (e,f ) Diptera: Calliphoridae, puparium and pupa within. Obtect adecticous pupae: (g) Lepidoptera: Cossidae; (h) Lepidoptera: Saturniidae; (i) Lepidoptera: Papilionidae, chrysa lis; (j) Coleoptera: Coccinellidae. ((a) After Evans 1978; (b, c, e, g) after CSIRO 1970; (d) after Chu 1949; (h) after Common 1990; (i) after Common & Waterhouse 1972; (j) after Palmer 1914)
The eggs adhere to the leaf surface because of a sticky secretion applied to each egg. (After Blaney 1976)
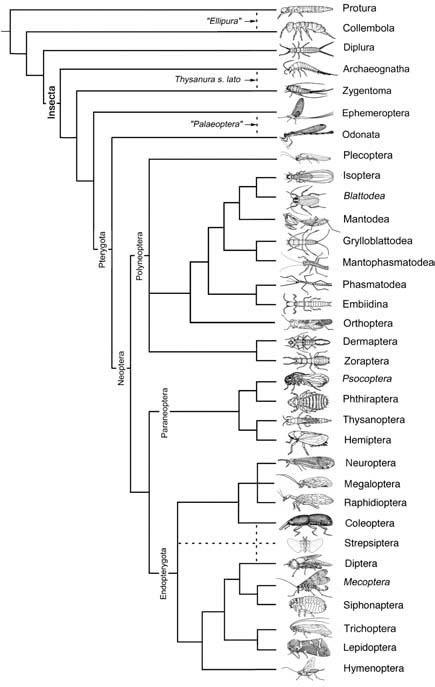
Broken lines indicate uncertain relationships. Thysanura sensu lato refers to Thysanura in the broad sense. (Data from several sources)