Alimentary Canal and Digestion
Insects feed upon many different kinds of food, including paper, wood, plant phloem and xylem sap, plant leaves, roots and stems, animal tissues, hair, wool, and vertebrate blood. The alimentary canal (often simply called the gut in much of the literature) evolved to accommodate such diverse foods in a variety of morphological and physiological ways. Thus, there is no “typical” insect alimentary canal just as there is no typical insect. Nevertheless, there are similarities in the structure of the alimentary canal in all insects and nearly all must digest some of the same complex molecules, such as proteins, lipids, and carbohydrates.
In every insect alimentary canal three regions can be identified morphologically and physiologically: the foregut or stomadeum, the midgut or mesenteron, and the hindgut or proctodeum. One or more of these regions may be greatly reduced in size, or expanded in size, depending upon the feeding behavior of the insect. A cuticular layer, the intima, attached to the epithelial cells, lines the fore- and hindgut regions. The old intima is partially digested and the residue sloughed off into the gut and excreted at each molt, and a new intima is secreted. The midgut does not have an attached cuticular lining, but may have a non-attached peritrophic membrane that separates the food enclosed within from the delicate surface of the midgut cells. If a peritrophic membrane is present, it is often secreted several times each day.
The Foregut
The buccal cavity (mouth), pharynx, esophagus, crop, proventriculus and attached salivary glands comprise the foregut. Secretions from the salivary glands attached near the mouth are swallowed with the food, lubricate the food, and may begin some carbohydrate digestion. In some insects the crop is not a noticeably modified part of the foregut, but often the crop comes off the foregut as a diverticulum. In other insects it is an enlarged portion of the foregut. In opportunistic and possibly irregular feeders such as praying mantids, the crop composes more than half of the alimentary canal, apparently an evolutionary development to store a large amount of food when available and tide the mantid over periods when prey is scarce. Some insects (for example, many orthopterans) regurgitate enzymes from the midgut into the crop, and these enzymes, along with salivary secretions, digest food in the crop. The digested food components still enter the midgut to be absorbed, and there is no evidence that the crop ever secretes enzymes itself. The cuticular intima creates a barrier even against the absorption of water from the crop. The proventriculus controls the entry of food into the midgut in liquid feeders, but in many insects it is modified into a grinding apparatus with hard, sclerotized ridges and spines, and heavy musculature for breaking and tearing the food into smaller particles.
The Midgut
The midgut in most, but not all, insects is the main site for digestion, absorption, and secretion of digestive enzymes. The epithelium is a single layer of cells, but several types of cells occur in some insects. The most common cells are tall, relatively narrow ones called columnar or primary cells by various authors. They have extensive microscopic microvilli on the apical or lumen surface and extensive invaginations of the basal cell membrane, features that greatly increase the surface area on both sides of the cell over which secretion and absorption occur. The columnar cells are the primary cells that secrete digestive enzymes and absorb digested products. In all insects that feed as adults and live for days or weeks, there are small regenerative cells distributed at the base of the columnar cells, or sometimes clustered together in nidi (nests). The regenerative cells grow into mature epithelial cells to replace cells worn out or those that disintegrate to release digestive enzymes. Midgut cells may be completely replaced every few days in insects that live longer lives. Gastric caeca, small finger or sac-like diverticula from the midgut, often arise at or near the origin of the midgut, but may be located at various points along the midgut. The caeca appear to secrete digestive enzymes and may be important in absorption of digested products.
The midgut does not have an attached cuticular lining on the surface of the cells, but midgut cells in the majority of insects secrete a thin membrane composed of chitin and protein, the peritrophic membrane, that surrounds the food and shields the delicate microvilli of the midgut cells from contact with potentially rough and abrasive food particles. Although the peritrophic membrane is thin, varying from 0.13 μm to about 0.4 μm thick, it also is thought to make it more difficult for viruses, fungi, bacteria, and protozoans to get to the surface of the midgut cells where they might be able to enter the cells and create an infection. Some insects produce several peritrophic membranes per day, each encasing the one before it, perhaps increasing protection from random breaks or punctures by larger food particles, and thus affording more protection for the midgut cells from possible fungi, parasites, viruses, and bacteria ingested with the food. A peritrophic membrane occurs in living representatives of some of the earliest insects to evolve, and it is believed to have evolved very early in a generalist scavenger feeder in which protection of midgut microvillar surfaces from food particles, sand, or other hard substances coincidentally ingested was likely to be important. A peritrophic membrane is present in many insects that do not feed upon rough or solid food, such as some blood feeders (but not all blood feeders), and in adult lepidopterans that take flower and plant nectars. Although the peritrophic membrane is not present in all groups of insects, like other gut features, it has been conserved over long evolutionary time, lending support to views that it has multiple functions, especially protection from disease invaders and may even have properties that could bind toxicants and limit their access to cells.
Absorption of digested food substances has not been studied in most insects, but one mechanism has been partially elucidated for absorption of amino acids derived from protein digestion in larvae of Lepidoptera. Interspersed among the tall columnar cells lining the midgut in larvae of Lepidoptera (and some other groups of insects as well) are cells shaped much like a goblet and called, appropriately enough, goblet cells. The apical cell membrane of the goblet cavity has metabolic machinery that uses energy derived from splitting ATP to push or pump protons (H+) into the goblet cavity. A different set of machinery in the goblet cell membrane, an antiporter mechanism, reabsorbs the protons and simultaneously secretes potassium ions into the goblet cavity. The net result of the secretion of potassium ions is that a strongly alkaline midgut (a midgut pH as high as pH 8 to about 11) is produced, and a high voltage (up to 240 mV in some reports) is created between the gut lumen (positive) and the interior of cells lining the gut. The voltage created by the pump enables an absorptive mechanism in membranes of columnar cells to reabsorb K+ and amino acids from protein digestion. Thus, potassium ions are recycled between the epithelium cells and the gut lumen and seem to play a major role in amino acid absorption. The high midgut pH may provide plant feeding insects some protection against tannins that are common in the food plants of phytophagous insects. Tannins can complex with an insect’s own enzymes and proteins in the food, and may result in reduced digestion and absorption. Many details and the precise metabolic components in the cell membrane that support and enable these secretory and absorptive mechanisms remain to be elucidated, but what is already known emphasizes the complexity of insect digestive functions.
The Hindgut
The hindgut is not only a posterior extension of the alimentary canal, but it also plays a major role in excretion through secretion of some substance into the lumen, and reabsorption of useful substances such as ions, water and some nutrients from the Malpighian tubule effluent. The Malpighian tubules typically arise at the origin of the hindgut (but exceptions do occur) and pass relatively large volumes of an ultrafiltrate of hemolymph components minus proteins into the beginning of the hindgut. The cuticular lining on hindgut cells is thinner and has larger pores than the lining in the foregut, permitting reabsorption of water, some ions, and useful metabolites that are returned to the hemolymph. In most terrestrial insects, water conservation is vital to life, and the hindgut must conserve the water that the Malpighian tubules flush into the hindgut. Waste products such as undigested food material (cellulose, for example, which most plant-feeding insects cannot digest and use), uric acid, and other allelochemicals picked up from the food are concentrated in the rectum and eventually excreted. The hindgut secretes some molecules into the lumen for excretion. Experimental evidence indicates that secretion and selective reabsorption helps regulate pH of the hemolymph in some insects. Specialized cells, the rectal papillae and rectal pad cells, in the rectum of many insects have characteristic ultrastructure and physiological mechanisms typical of highly reabsorptive cells. Water conservation by the rectum results in the relatively dry frass or fecal pellets characteristic of many terrestrial insects.
The highest degree of specialization in the hindgut occurs in those insects that digest cellulose, such as termites. In termites, the hindgut is usually divided into several chambers harboring either bacteria or protozoa that secrete all or part of the cocktail of enzymes needed to digest cellulose. Glucose, liberated from cellulose digestion, may be fermented by the resident microorganisms, with the end products being short chain fatty acids (principally acetic acid) that can be absorbed by the termite and used as an energy source. Some termites release methane, a greenhouse gas, from the metabolic activities of their microorganisms, but whether this is a significant natural source of methane is a topic of debate by various scientists.
Digestive Enzyme Secretion
Midgut cells secrete and release digestive enzymes in several ways. They may enclose digestive enzymes in small vesicles surrounded by a membrane and then release the enzymes into the alimentary canal by fusing the vesicle membrane with the cell membrane. In some insects, parts of the cell (some of the microvilli) or the entire midgut cell may disintegrate and release enzymes into the gut lumen. Of course, when the entire cell breaks down, the cell must be repaired or replaced. Replacement occurs through the growth of the regenerative cells.
Extraoral digestion (digestion outside the insect body) occurs in some insects, including seed feeders and some predatory insects. By injecting enzymes from the salivary glands and midgut into the food source (animal or plant material) and then sucking back the liquefied digestion products, insects can utilize very high percentages of the nutrient value of the food source. Some insects reflux enzyme secretions and partially digested products by repeatedly sucking up reinjecting the liquefied juices into the food. Ref luxing mixes the secretions and fluids extends the effective life of the digestive enzymes, and is particularly effective when the food contains a limiting boundary, such as the shell of seed or the cuticle of an insect that acts as a container for the liquefying body contents.
Carbohydrate Digestion
Starch and sucrose are the typical carbohydrates that insects digest from plant food, and glycogen and various sugars are present in animal tissue eaten by carnivorous insects. Cellulose, the major complex polysaccharide present in plant tissue, cannot be digested by most insects. Carbohydrate digestion begins with the action of α-amylase, an enzyme present in the salivary gland secretions of many insects. Amylase works best at slightly acid pH, and hydrolyzes interior glucosidic linkages of starch and glycogen, resulting in a mixture shorter dextrins. In the midgut α-glucosidase and oligo-1,
Lipid Digestion
The major storage forms of lipids (fats) in both plants and insects are triacylglycerols, esters of fatty acids with glycerol. Midgut cells, and in some cases symbionts, secrete lipases, which are enzymes that hydrolyze triacylglycerols and release fatty acids and glycerol. Amino acids, proteins, and fatty acylamino complexes act as emulsifiers in the midgut of some insects facilitating the digestion of fats. The glycocalyx layer, a viscous protein and carbohydrate complex that often lies on the surface of the microvilli, probably aid in emulsifying fats and in promoting contact between lipases and triacylglycerols. Fatty acids released from triacylglycerols are resynthesized into the insect’s own triacylglycerols and stored in fat body cells. Immature insects typically store relatively large amounts of triacylglycerols, and use some of the released fatty acids during pupation and for egg development. Some insects, for example Orthoptera, Lepidoptera, and some aphids, mobilized fatty acids rapidly enough to use fatty acid metabolism to support flight, but other groups such as Diptera and Hymenoptera cannot release fatty acids from the fat body and transport them to the flight muscles rapidly, and so they only use carbohydrates for flight energy. They still can use lipids during pupation, and for other metabolic processes that occur more slowly.
Protein Digestion
All animals must have a pool of amino acids available for synthesis into proteins, and for repair of tissues and organs. Most animals, including insects, get their amino acids from digestion of dietary proteins. Within insects as a group, there are several different types of protein digesting enzymes, some of which act at acid pH, others at slightly alkaline pH, and some at highly alkaline pH. Usually a particular species will have several different proteinases, but no insect is known to have both an acid-effective proteinase and an alkaline-effective proteinase. The pH of the alimentary canal is important to the action of any digestive enzyme, and no insect is known to have an alimentary canal that is strongly acid in one part and strongly alkaline in another part.
Proteinases are classified broadly as serine, cysteine, aspartic acid, and metallo-proteinases depending upon the amino acid or metal at the active site of the enzyme. Trypsin and chymotrypsin are two endoproteinases with alkaline pH optima (about pH 8) that are common in many insects and which attack large proteins internally at the linkage between certain amino acid, thus breaking the protein into smaller polypeptides. Most insects appear to have several types of exopeptidases that remove the terminal amino acid from a protein or peptide chain. Thus, through the concerted action of both types of digestive enzymes, a protein can be completely digested with release of its component amino acids. Cysteine- and aspartic acid-proteinases have mildly acid pH optima, and are called cathepsins by some authors. All members of a taxonomic group may not have the same type of proteinases. Many beetles have cysteine proteinases most active at slightly acid pH, while some scarabeid beetles secrete serine-proteinases that act at the high midgut pH typical of these insects, and they have no detectable cysteine-proteinases. Lepidoptera typically secrete trypsin-like enzymes active at alkaline pH.
One defense mechanism that has evolved against herbivory in many plants is the presence of proteinase inhibitors, some of which inhibit serine proteinases while others act upon cysteine proteinases. Experimentally, it has been shown that some insects secrete multiple trypsin enzymes (isozymes of trypsin) and others just secrete larger amounts of the same few isozymes aſter consuming a trypsin inhibitor. This counter action by the insect probably allows some protein digestion to escape ingested inhibitors, but transgenic plants designed to have proteinase inhibitors have been tested and proven to have adverse effects upon the growth of some insects.
Absorption of Digested Products
Few details are known about the absorption of digested products by insects. In many vertebrates, glucose absorption from the alimentary canal requires an active mechanism involving ATP to supply the energy. In those insect that have been studied, glucose from digestion of carbohydrates is rapidly absorbed passively by a process known as facilitated diffusion, and involvement of ATP is not necessary. Fat body cells on the hemolymph side of the gut rapidly synthesize absorbed glucose into the disaccharide trehalose, keeping the hemolymph con centration of glucose low in most insects. Consequently, even low concentrations of glucose in the gut have a favorable diffusion pathway to the hemolymph and continue to be absorbed passively. In larvae of a few lepidopterans that have been carefully studied (Manduca sexta, Philosamia cynthia, Bombyx mori), amino acids are actively absorbed by transport proteins in the apical membranes of midgut columnar cells. Energy for absorption comes from the high K+ concentration in the gut lumen and high transepithelial potential created by the proton- ATPase pump active in midgut goblet cells. The transport proteins in these membranes show strong specificity for particular amino acids, and transport systems for at least six different amino acids are known, and transport systems for other amino acids probably will be discovered. In the Colorado potato beetle, Leptinotarsa decemlineata, transport proteins for leucine and tyrosine have been demonstrated in midgut tissue.
Gut pH
The pH of the alimentary canal is highly variable in different species of insects. The pH of a gut segment influences the action of enzymes secreted into or carried with the food into the gut, influences solubility and toxicity of toxins and plant allelochemicals, and may alter the population of gut microorganisms. In most insects, the crop has little or no presence of buffering agents and tends to be slightly acidic, a factor favoring carbohydrate digesting enzymes. Larvae of Lepidoptera and Trichoptera tend to have a very high midgut pH, varying from about 8 to 10, promoted by goblet cells that secrete potassium and bicarbonate into the lumen of the midgut. They have protein digesting enzymes that are favored by the high pH, and the high pH may afford some protection from tannins and other allelochemicals that they ingest with their plant food.
Illustrative Examples of Diversity in Food, Form and Function of the Alimentary Canal
The following examples are not intended to be a comprehensive review of foods, and alimentary canal structure and physiology, but will merely highlight interesting diversity.
Opportunistic feeders may have evolved modifications to capture and store food when available, and thus survive lean periods when food is not available. For example, the foregut of the praying mantis, Tenodora sinensis, is long and wide, and occupies nearly the entire length of the body, apparently an adaptation for storage of prey when it can be captured. The midgut, eight gastric caeca, and hindgut are compressed into the last three abdominal segments. Probably much of the digestion occurs in the posterior part of the crop with enzymes passed forward from the small midgut.
Considering the universal presence of cellulose in plant tissues, relatively few insects evolved the ability to use cellulose as a source of nutrients.
Termites, some beetles, a few hymenopterans, and a few cockroaches do use cellulose as a carbohydrate source. The hindgut of termites is highly specialized for housing gut microbiota that provide the cellulase enzymes needed to digest cellulose, although there is some evidence that certain termites may be able to produce some or all of the several enzymes necessary to completely digest cellulose. Gut variation exists among the castes in a colony; for example, soldiers in the family Rhinotermitidae are fed liquid food by the worker caste, and do not have to digest cellulose so they have reduced gut structure. The workers are responsible for colony construction and nutrition, and they have highly evolved hindgut chambers to hold various types of microbiota. Termites hatch without their gut microbiota, and lose most of their gut symbionts at each molt, but they become reinfested by feeding upon fluid and excreta from older nymphs. Termites in the family Termitidae have symbiotic bacteria in the hindgut, while termites in some other families have flagellate protozoans as well as bacteria in a multi-compartmented hindgut, and they get some or all of their cellulase(s) from their symbionts. Some termites, the Macrotermitinae, cultivate fungus gardens in their underground nests and get their cellulases from the conidiophores of the fungus. Symbionts in the hindgut of some termites can capture atmospheric nitrogen in an organic form, which is probably quite important to many termites because their diet of wood is relatively low in proteins. Some fungus-growing termites convert some of the protons (H+) and carbon dioxide from the initial fermentation of glucose into methane (CH4), and some investigators have suggested that termites are a significant environmental source of methane, a greenhouse gas.
Larvae of the woodwasp (Hymenoptera, Symphyta, Siricidae, genus Sirex) acquire cellulase and xylanase from fungi ingested with the wood on which they feed. Larvae of cerambycid beetles and of some other beetles feed upon wood in down or dying trees; they generally have long life cycles because wood is so nutrient poor, especially protein poor. Fungi growing in the dead wood and ingested by the larvae may provide additional nutrients and/or enzymes for digesting the wood.
Hemiptera take xylem or phloem sap, both of which are poor in amino acids and protein, but usually rich in sucrose (150 to more than 700 mM). They typically excrete a copious, dilute fluid, and in some, such as aphids, the fluid contains so much sugar that it is called honeydew. They have to ingest large volumes of fluid to get the amino acids, and then they have to get rid of the excess water and sucrose. A characteristic evolutionary feature of the gut in Hemiptera is the filter chamber in which a loop of the hindgut is in direct contact with part of the foregut and a great deal of the ingested fluid diffuses directly into the hindgut from the foregut without passing through the midgut. This, of course, causes loss of some amino acids and other components that may be needed, but water and sucrose, both of which are in excess of needs, are the major components lost. The filter chamber is able to concentrate gut fluid up to
Pre-oral digestion with enzymes secreted into the prey occurs in many of the predacious beetles. Seed feeders also employ pre-oral digestion by injecting salivary secretions and possibly regurgitated midgut enzymes into the seed, allowing these to liquefy part of the seed, and then sucking the nutrients and enzymes back. Pre-oral digesters often reflux the liquefied contents by repeatedly imbibing and then reinjecting the mixture of enzymes and digested nutrients into the seed. Refluxing likely conserves enzymes longer, gives them more opportunity to function, and allows the insects to use more of their potential food.
In honeybees and some other related hymenopterans, the midgut is closed off from the hindgut by a plug of cellular tissue during larval development, and any food that cannot be digested and absorbed into the body must remain in the midgut. Just before pupation the connection between midgut and hindgut is opened, and accumulated undigested residue, such as the shells of pollen grains, is excreted into the cell. Adult honeybees clean the cell and the larva pupates inside the cell.
Nectar taken by male and female mosquitoes is stored in a large, sac-like crop that is a diverticulum from the foregut, but blood meals taken by the females are passed directly into the midgut for the beginning of digestion. The midgut is differentiated functionally into an anterior and a posterior region. The anterior part secretes carbohydrate digesting enzymes, and nectar components are digested as fluid from the crop and is passed into the anterior midgut. This arrangement keeps possible trypsin inhibitors that may be present in nectar away from the site of protein digestion, which occurs in the posterior midgut. Simple sugars resulting from digestion, or those already in the nectar, are absorbed in the anterior midgut. The posterior midgut cells secrete trypsinlike enzymes and protein (blood) digestion and absorption occur in the posterior midgut. The posterior midgut cells, more so than anterior midgut cells, have extensive microvilli and basal infoldings characteristic of secretion and absorptive processes. The midgut cells in this region get stretched by the large volume of blood that a mosquito takes if it is allowed to feed to repletion. Consequently, the cells have several types of connecting structures (desmosomes) between cells to help hold them together and prevent excessive leaking of materials in or out between cells while they are stretched.
Larvae of Lepidoptera have a very short foregut, a large, long, relatively straight midgut, and a short hindgut. There is no storage or digestion in the short, nearly vestigial foregut. Nearly all lepidopterous larvae are phytophagous feeders, and the gut modifications appear to be an adaptation to pass food quickly into the long midgut so that digestion can begin. Feeding is nearly continuous when plenty of food is available, and larvae may ingest more than their body weight in food daily. Food moves rapidly through the relatively straight gut and frass droppings are frequent in phytophagous caterpillars. Because the larval and adult forms of Lepidoptera have very different life histories and food habits, the adult gut is quite different from that of the larva. Many adult Lepidoptera feed only upon nectar, which is stored in the crop and slowly released into the midgut for digestion to simple sugars. Some adult Lepidoptera have vestigial mouthparts and do not feed at all; they survive and (females) produce eggs at the expense of body substance, and they generally live only a few days. An unusual food utilized by Tineola bisselliella larvae (clothes moth) is wool, and larvae have a very strong reducing action in the midgut that breaks disulfide bonds between adjacent loops of the proteins, causing the wool proteins to lose their three-dimensional shape and unfold. This allows more access for protein digesting enzymes.
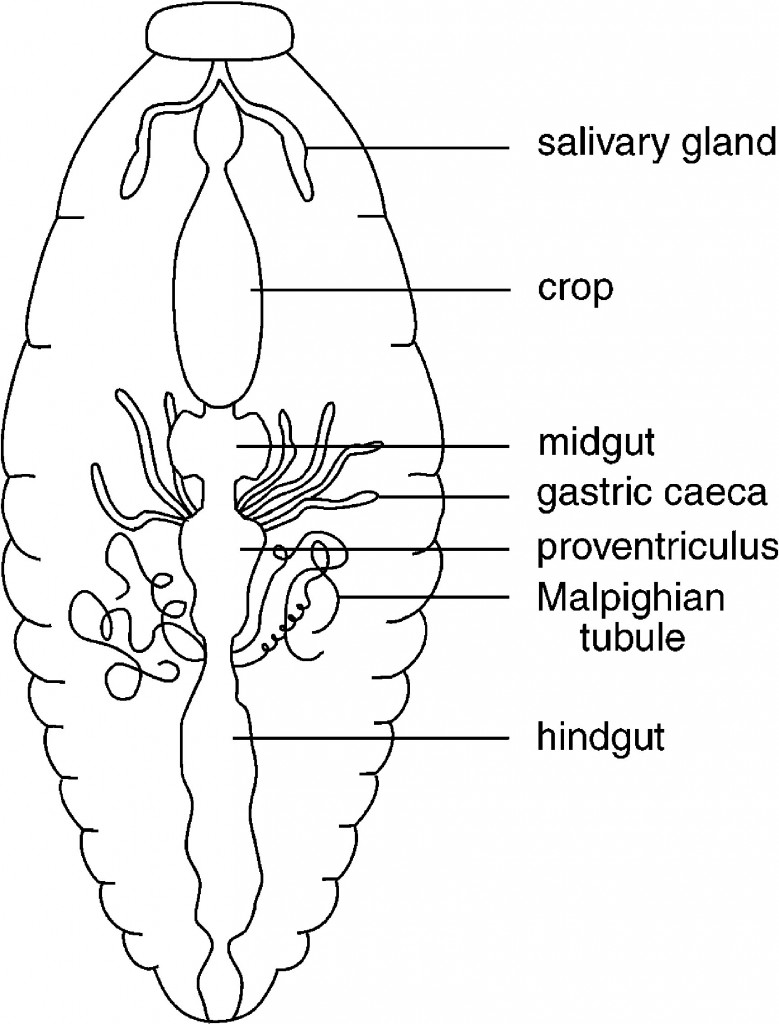
A generalized drawing of the alimentary canal in a cockroach to show the major divisions of the canal.
Many variations occur in the overall structure of the alimentary canal in insects, and this is not intended to suggest that the cockroach alimentary canal is typical of insects.
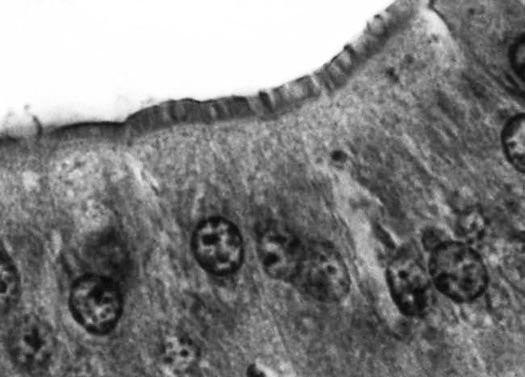
A brush border of microvilli on the lumen surface of midgut cells in a mole cricket.

Alfalfa (Lucerne) Pests and their Management