African Pine-Feeding Grasshoppers
These grasshoppers are excellent examples of indigenous insects that developed a preference for an exotic plantation-grown crop, in this case pines. Plagiotriptus pinivorus attained some prominence aſter causing persistently severe defoliation of exotic pines, especially Pinus patula, in Malawi in the 1960s, resulting in significant tree mortality. A smaller scale defoliation of P. patula was observed at Morogoro in Tanzania in the mid 1980s, which was attributable to another, very closely related species, Plagiotriptus hippiscus. Both of these grasshoppers are highly polyphagous, including herbaceous hosts, shrubs and both angiosperm and gymnosperm trees. The prime requirement for P. pinivorus seems to be access to evergreen or semi-evergreen vegetation in areas of moderate to heavy rainfall, i.e., mostly at altitudes between
Plagiotriptus pinivorus in Malawi exhibits three generations every 2 years, and the complete life cycle takes about 1 year. Nymphs and adults have been observed on pines throughout the year, except from December to late January. Copulation occurs anytime, but peaks from October to January and May to June. During copulation, the small male assumes a characteristic dorso-lateral position by clinging to one of the hind femurs of the female. Both males and females are promiscuous. About
Eggs incubate from
Nymphal peak emergence and rainfall are strongly correlated in February, allowing prediction of emergence two weeks in advance. Another smaller peak in emergence in August, however, cannot be explained by rainfall. The first instar nymph is ephemeral (about 12 h), and will molt immediately when reaching the soil surface, before feeding on ground vegetation for the next
Adult males (Fig. 25) are about
Numerous invertebrate and vertebrate predators, including skinks, birds and blue monkeys, as well as parasites were documented, but ultimately they were deemed insufficient by themselves to reduce populations of the grasshopper to nondamaging levels. As a result, sticky bands and chemical controls were relied on for monitoring and control purposes, respectively. In the 1960s in Malawi, gamma-BHC at 0.5% proved the most effective insecticide for ground and aerial applications at ultra-low volume formulations. Spraying of road banks was particularly recommended, as insects clustered there for oviposition in the bare ground. present; labial palpi long and porrect,
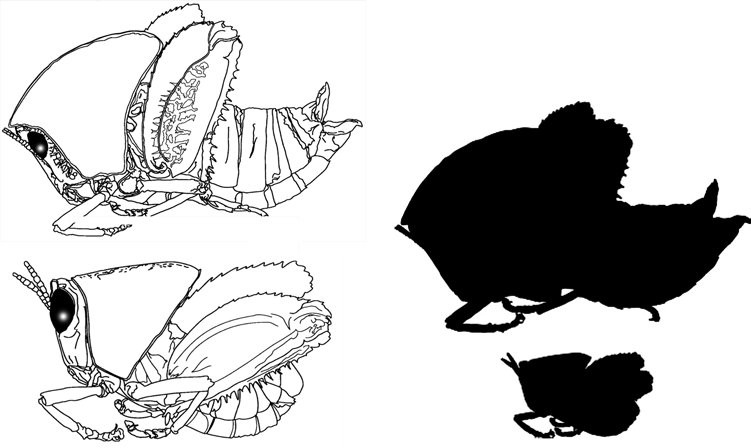
Figure 25 Plagiotriptus hippiscus female (above) and male (below) with size comparison (shaded)

African Maiden Moths


