Acoustical Communication in Heteroptera
Acoustic signaling is found in many hemipteran families. It serves a variety of purposes, particularly defensive behavior such as repelling potential predators and signaling alarm or distress, but also for species spacing within a particular habitat, reproduction, and coordination of group actions. Vibratory signals for reproductive purposes may be produced by males and/or females, leading to aggregation to mate attraction, courtship and copulation. Non-receptive females may sing to reject copulation (male-deterring stridulation), as in the subfamily Triatominae (Reduviidae) and in Pentatomidae (e.g., Nezara viridula).
The vibratory signals produced by many insect species cannot be heard by the human ear because the sounds are low frequency and generally transmitted by mechanical vibrations through the substrate, and not by the air. The study of acoustical communication has greatly progressed in accordance with improvement of recording and analyzing equipment, including the necessary computer soſtware.
Production of Vibrational Signals and Songs
Vibrational signals and songs are produced by stridulation (stridulatory device, stridulatory organs), by body vibration, or by a simple tymbal mechanism. Stridulation occurs widely in Heteroptera, and is the act of producing sound or vibration by rubbing together certain body parts. The first systematic survey of sound-producing devices in the Heteroptera was that of Handlirsh in 1900. To stridulate, usually both a movable and a stationary portion are needed. The movable part is called the plectrum or scraper. The stationary portion may be called the stridulitrum, file, strigil (strigile, strigilis) or lima (pl., limae). The stridulitrum is typically striated or finely tuberculated, and the plectrum is a structure with a well-defined lip or ridge, tubercles, or provided with spines.
Different parts of the body may be involved to function as the stridulatory device. The forewing edge is most commonly used as a stridulitrum (file), while the hind femur is the most usual structure used as a plectrum (scraper). Other stridulitrum may be located in the head, associated with the mouth (labium, maxillary plate), the thorax (propleurum, metapleuron, prosternal groove), the wings (metathoracic wing vein, hypocostal lamina or articulatory sclerite, underside of clavus), the legs (forecoxa, mesotrochanter, tibia, femora), and the abdomen (sternum, the connexival margin, posterior margin of the pygophore). There are also a variety of locations for the plectrum or scraper: the rostrum, the legs (forecoxal cavity, coxal peg, hind tibia, and fore-, middle or hind femur) or the abdomen. Some stridulatory devices are present in several families, whereas others are only known from a few genera or even a single species.
Examples of stridulation devices are that of the Corixidae (spinose area inside the front femur against the clypeus, genitalia against abdomen segments), Scutelleridae (wart-like, toothed tubercles in the hind tibia against the femur), Reduviidae (tip of the labium against a cross-striated furrow (Fig. 8) in the prosternal groove), some Pentatomoidea and Lygaeiodea (dorsal abdominal files against teeth on the under-sides of the hindwings), some Miridae, Lygaeidae, Largidae and Alydidae (hind femur against forewing edge) and some other Pentatomoidea (tubercles on the hind femora against strigose regions on abdominal sterna).
Morphological differences in the stridulatory device may, in some cases, be related to differences in the songs emitted by either males or females, as occurs in the burrower bugs (Fig. 9) Scaptocoris castanea and S. carvalhoi. In S. castanea, males have a longer stridulitrum than females. However, in S. carvalhoi the stridulitrum length does not differ between sexes. Instead, in S. carvalhoi the male stridulitrum has more teeth than in the female stridulitrum. There are no intersexual differences for this latter morphological trait in S. castanea.
Differences in the stridulatory apparatus may be related to interspecific differences, with a specific diagnostic value. In the genus Triatoma (Reduviidae), it is possible to distinguish between T. guazy and T. jurbergi at any nymph stage or in the adults by studying the stridulatory sulcus (stridulitrum). As T. jurbergi is naturally infected with Trypanosoma cruzi, the causal protozoan of Chagas disease (American trypanosomiasis), identification of specimens along their whole life cycle is of great medical importance.
In other instances, morphological differences in the stridulatory device do not cause differences in their song patterns. For example, in Reduviidae of the subfamily Triatominae, individuals of the same species have stridulatory grooves with different inter-ridge distances, though the frequency spectra and repetition rates are similar.
A tymbal is formed by abdominal tergal plates fused together and which vibrate over a hollow chamber within the abdomen. The tymbal is activated by muscular contractions and produces body vibrations that are low frequency. Tymbals have been found in Piesmatidae, Pentatomidae, Acanthosomatidae, Cydnidae, Lygaeidae, Coreidae and possibly Reduviidae, and similar vibration-producing mechanisms have been found in Plataspidae and Rhopalidae. Differences exist about precise abdominal parts and muscle contraction mechanisms among the tymbals of different families. For example, the description of the N. viridula tymbal follows: The first and second abdominal tergites are fused into a forward-backward movable tymbal-like plate that is loosely fixed, anterior and posterior, to the thorax and to the third abdominal tergum, by a chitinous membrane, and more firmly, laterally, to the pleurites. Longitudinal and lateral compressor muscles contract synchronously and in phase with these vibratory waves.
All Heteroptera species investigated so far emit low frequency narrow-band signals by body vibration, and/or broadband signals produced by stridulation. For example, in Cydnidae and Pentatomidae, vibratory mechanisms produce a low frequency vibration (around 100 Hz), and the stridulatory vibration extends up to 10 kHz.
Reception of Vibrational Signals and Songs
Although sound production is found quite widely in Heteroptera, it is not common to find structures specialized for sound reception. Sound reception in Heteroptera is possible due to the presence of either scolopophorous organs or tympanal organs.
Scolopophorous organs are mechanoceptors, and they occur widely in insects. They are composed of sensory sensilla (scolopodia), which may be arranged in groups, and are distally attached to a membrane in the body wall or to the body wall itself. Scolopophorous organs may be located in antennae (Johnston’s organ), legs (subgenual organ, joint chordotonal organ), thoracic pleura, or abdominal terga.
Legs are the site of sensory organs that detect vibratory signals with highest sensitivity. For example, at the dorsal side of each leg of N. viridula there are four scolopodial organs: femoral, tibial, tarsopretarsal and subgenual organs. The receptor neurons may be low frequency (most sensitive between 50 and 100 Hz) or high frequency sensitive (there are two types: middle frequency neurons being sensitive around 200 Hz, and higher frequency neurons sensitive around
Also, the Johnston’s organ of N. viridula has several vibratory sensitive organs which respond in the frequency range between 30 and 100 Hz. When standing on its host plant, the male of N. viridula, by different combinations of legs and antennae, may compare the vibratory signals on two branches of the host plant, and choose the best one in order to locate the singing female. The most probable mechanism underlying resolution of direction by vibratory cues (vibratory directionality) may be time-of-arrival differences (perception of vibratory signals by two different receptors in the insect). For example, when legs of the receptor bug are separated by 2 cm, a time-of-arrival difference between
0.125 and 0.250 ms is created, very close to that found in scorpions, where vibrational directionality is well known. Reduviidae also receive vibratory signals via legs and antennae. Fewer data are available on leg vibratory receptor organs in other land bug species. Among land bugs, no sensory organs for airborne sound have been found.
Tympanal organs have been found in the mesothorax of the Corixidae, and are in contact with the physical gill air bubble. They are able to catch airborne sounds, and to respond to stridulation frequencies produced by conspecific bugs.
Transmission of Vibratory Signals and Songs
Independent of their mode of production, vibrational signals may be transmitted by the substrate (substrate-borne vibrations) or by the air (airborne vibrations). The signals may travel a short or long distance, or travel at a low or high speed. Low-frequency components are more suitable for longer-range communication through plants. Low frequency signals travel longer distances, but slowly; high frequency signals travel shorter distances, but quickly. Long-range vibratory songs are associated with pre-mating calling and vibrational orientation, and close-range vibratory songs are associated with courting rivalry and repelling.
Substrate-borne vibrations are less costly to the emitter. Also, substrate-borne vibrations are more far-reaching signals for intraspecific communication and not easily perceived by a potential predator or parasitoid. Usual substrates to transmit vibrational signals are plants or soil.
The characteristics of plants as transmission media for insect-produced vibrations have been described, and in many respects they determine signal production and the mode of reception. Depending on the physical properties of the host plant, the vibratory signals are transmitted effectively or not. Vibrations can be transmitted all along the stem, but the physical properties of a plant (e.g., elasticity, water content) affect resonance of insect vibrations. For transmission through plants, insects commonly emit broadbanded-mixed stridulatory and vibratory signals. Higher-frequency signals produced by stridulation are less relevant for longdistance communication through plants. However, narrow-band and low frequency songs are efficient in long-distance communication when well tuned to the resonant spectra of their host plants. The vibrational pulse reflects when attaining both the root area and top of the plant, and reflected waves travel up and down the stem several times. Reflections change the patterns of the input signal. Abiotic factors (temperature, rain, wind) may significantly modify plant resonance, masking insect vibratory signals and thus the effectiveness of the signal. Plant-borne vibrations seem to be important in the success of group-living, herbivorous insects for locating and remaining in a group of conspecifics, for locating food resources, and to avoid predation. Also, small insects that are not able to emit airborne sounds efficiently at low frequencies in many cases communicate with vibratory signals transmitted through plants.
In the stink bug Nezara viridula (Pentatomidae) (Figs. 10 and 11), a species which has become a model for all Pentatomorpha in relationship to acoustical communication, its vibratory signals were recorded and described first as airborne sound. However, further investigations showed that their most impor tant mode of transmission is as substrate-borne vibrations. In N. viridula, a male could perceive a female calling in a Cyperus stem 2 m away from him, mechanically coupled only by roots and the surrounding earth. Below the leg of the singing bug, the intensity of signals was about 4 mm s—1. On the bottom of the same stem (a distance of 80 cm) it decreased to around 3 mm s—1, and at the place of the receiving bug, 200 cm away, it was approximately 0.5 mm s—1.
When transmitted through the soil, signals travel a shorter distance and are more attenuated than when transmitted through a plant stem. For example, in Scaptocoris species (Cydnidae) the velocity of soil transmitted signals varied between 1.5 and 12.9 ms-1 at a distance of 0.5 cm.
Acoustic Characteristics of Vibrational SignalsA vibrational signal may be characterized by its temporal (pulse train duration, repetition times, inter-pulse intervals) (Fig. 12) and spectral characteristics (dominant frequency). All of these characteristics may be measured by the receptor insect, who may modify its behavior in response to the message. The dominant frequency of signals produced by the vibratory mechanism lies between 50 and 200 Hz in most Heteroptera. Between species, songs differ in their time structure and amplitude modulation of their units. On the other hand, spectrally and temporally different pulse trains trigger the same male behavior in N. viridula.
In Rhodnius prolixus (Reduviidae), the maledeterring call has a main carrier frequency of about 1500 Hz, and the disturbance stridulation has a main carrier frequency of about 2200 Hz. In Rhinocoris iracundus (Reduviidae), low-frequency components of carrier frequency below 200 Hz are exchanged with frequency-modulated stridulatory components whose dominant frequency lies between 1 and
2 kHz. In Triatoma infestans (Reduviidae), distress songs have a peak of frequency between 700 and 800 Hz, although in other reduviids the carrier frequency may reach about 2000 Hz.
In N. viridula, dominant frequencies between 80 and 150 Hz were found in songs either as airborne sounds, or substrate or body vibrations. Body vibrations are around 100 Hz, and lie close to the range of best frequency sensitivity of low frequency receptor cells.
Specificity and Variability of Vibratory Signals
Sounds, especially those involved in the reproductive process (attraction, courtship, copulation), can be very complex and highly species-specific, and may be used as taxonomic characters of land bugs. In contrast, signals are much less specific when they provide information about enemies, rival mates, or serve as distress (disturbance or alarm) signals.
Females of N. viridula sing to trigger the male approach, and to evoke emission of the male courtship song. Females coming from populations of different geographic origin emit different calling songs, which can be differentiated by males. Females of N. viridula may emit a song that rejects copulatory attempts of males and stops their courting, and this is also known in the reduviid Rhodnius prolixus. The courtship songs of both males and females in different populations are not markedly different, but the calling songs may differ in some features and may be the source of reproductive isolation among populations. Nezara viridula produces four different species and sex-specific songs, and two of them play a vital role in mate location. There is song variability within populations (inter-individual variability). To assess those differences, the temporal song (pulse train duration, repetition times, inter-pulse intervals) and spectral song characteristics (dominant frequency) may be measured. Males usually show a preference for the females of their own population, although they may recognize females from other populations as potential partners.In Tritoma infestans, stridulation songs differ in their syllable durations, repetition rate and main carrier frequency, depending on the song function. Differences come from rubbing their rostrum (scraper) at different speeds on the prosternal file. Also in Rhodnius prolixus, the different frequency between deterrent and disturbing signals can be explained on the basis of a different rubbing velocity by the proboscis against the prosternal stridulatory organ (Fig. 13).
In Scaptocoris carvalhoi and S. castanea (Cydnidae), two sympatric burrower bugs, high individual variation of the dominant frequency was observed in both male and female emissions (Fig. 14).
Vibratory Signaling in the Families of HeteropteraVibratory signaling has been reported in several Dipsocoromorpha and Leptodomorpha, but has been better studied in the following families: Veliidae (Gerromorpha), Nepidae, Corixidae, Notonectidae (Nepomorpha), Reduviidae, Miridae, Tingidae, Nabidae (Cimicomorpha), Aradidae, Acanthosomatidae, Cydnidae, Pentatomidae, Scutelleridae, Tessaratomidae, Thaumastellidae, Colobathristidae, Lygaeidae, Piesmatidae, Largidae, Alydidae, Coreidae, Rhopalidae (Pentatomorpha) (Figs. 15 and 16). Selected examples follow:
Corixidae
In Corixidae, males or both sexes use speciesspecific sound for mate attraction and in courtship. The sounds are produced by stridulation, i.e., rubbing together specially modified parts of the body, or the partner’s body.
Notonectidae
In Notonectidae, males produce species-specific courtship sounds by rubbing roughened parts of their front tibiae and femora against a special stridulatory region at the base of the rostrum (Fig. 17). In genus Buenoa, the sound can be heard at a distance of several meters. While next to the female, but before clasping her, the sound pattern can change.
ReduviidaeDistress (disturbance or alarm) signals in Reduviidae may be produced either as nymphs or adults (males and/or females). In Panstrongylus rufotuberculatus (Reduviidae), stridulation occurs only under conditions of extreme provocation. Its sound is audible by the human ear, which is unusual among stridulating Triatominae, and is similar to the sound of sandpaper scraping wood. The tip of the rostrum is rubbed along the transversely ridged prosternal groove with an anterior-posterior movement; the return stroke (posterior-anterior) is silent. Stridulation lasts for about 5 min, although the insect remains immobile when held for a longer time. In a silent environment, the sound is audible at about 1 m away. A disturbance call has been described in the following triatomine species: Dipetalogaster maxima, Triatoma infestans, T. guasayana, T. sordida, Panstrongylus megistus and Rhodnius prolixus.
In the spined assassin bug, Sinea diadema, agonistic interactions between adult females may be resolved by stridulation in 33% of the cases. Stridulating individuals retreated more oſten than their non-stridulating opponents, indicating that stridulation may be a startle mechanism employed by temporarily disadvantaged individuals to escape from encounters. Together with other signs, stridulations provide information on the identity and relative fitness of the opponent.
The acoustic repertoire of the ambush bug, Phymata crassipes, is quite large and may be displayed by females, males or nymphs. Its vibrational songs may be emitted in response to, and alternating with, calls from conspecifics, or even human speech or whistle. Sound emission is related to disturbance, interaction with other males and females, or courtship. Signals are produced by locomotory, stridulatory and/or vibratory mechanisms. Airborne signals directly or indirectly stimulate vibrational receptors. Bugs within a group respond to each other only via substrate, even in close proximity.Miridae
Although a stridulatory device has been described in several tribes and subfamilies, the functions of acoustical communication in Miridae are still unknown.
Tingidae
In the tingid Corythuca hewitti, vibrational signaling during group movements may occur as groups of nymphs are attended by a female. It has been reported that a disturbance of the leaf where
C. hewitti aggregated caused feeding to stop and dispersal by the bugs, with occasional stopping of the bugs to vibrate the abdomen in a vertical plane, a behavior followed by conspecifics.
Cydnidae
In cydnids, the low species and sex specificity of pure stridulatory signals indicates that these vibratory emissions may play a role in disturbance (defensive) behavior, as in Tritomegas bicolor. Stridulatory signals are also related to aggregation or some other unspecific behavioral context. Cydnid bugs engage in rival singing and also distress (disturbance or alarm) signals, either as nymphs or adults (males and/or females).
Courtship, acceptance and rivalry songs show higher specificity and in most cases are produced by low frequency body vibration and/or by stridulation. Tritomegas bicolor produces courtship, mating, and male rivalry calls by stridulation and body vibration. In Sehirus luctuosus, the male’s courtship call is produced by body vibration, giving a drumming song. Two types of speciesspecific male courtship songs, produced by stridulatory and vibratory mechanisms, have been described. The first type triggers female response, a species-specific agreement song. The second type stimulates pair formation.
In the group-living species of genus Scaptocoris, the absence of low frequency components of the emitted signals and the uniformity of songs indicate that calling and courtship may be mediated by signals of other modalities. The lack of low frequency signals may be explained by the direct contact of the bug with soil, which mechanically prevents free vibration of the abdomen.
PentatomidaePentatomid bugs engage in rival singing. For example, Nezara viridula and Rhapigaster nebulosa may alternate rival songs until one or both stop singing, and in P. lituratus, males perform rival singing. Vibrational directionality has been demonstrated in host or prey searching in the predatory stink bug Podisus maculiventris.
The general pattern of singing during premating behavior is similar for all Pentatomoidea. Calling starts with the emission of the female calling song, which triggers males to respond with calling and courtship songs, activates them to walk on the plant, and enables directional movement toward the female. Alternation of male and female songs may result in more or less complex duets, as is the case in N. viridula. Nezara viridula vibrates its body as part of intersex communications (courtship, directional cue for locating the mate, mate recognition), which implies that substrate-borne signals are highly species-specific. The female song causes the male to walk, to respond with the calling and courtship songs, and to approach the source of the song with characteristic search behavior. In contrast, females show no reaction to vibratory stimulation and no vibrational directionality.

Figure 8 The stridulatory apparatus in Reduviidae: the tip of the labium is rubbed against a cross-striated furrow in the prosternal groove.
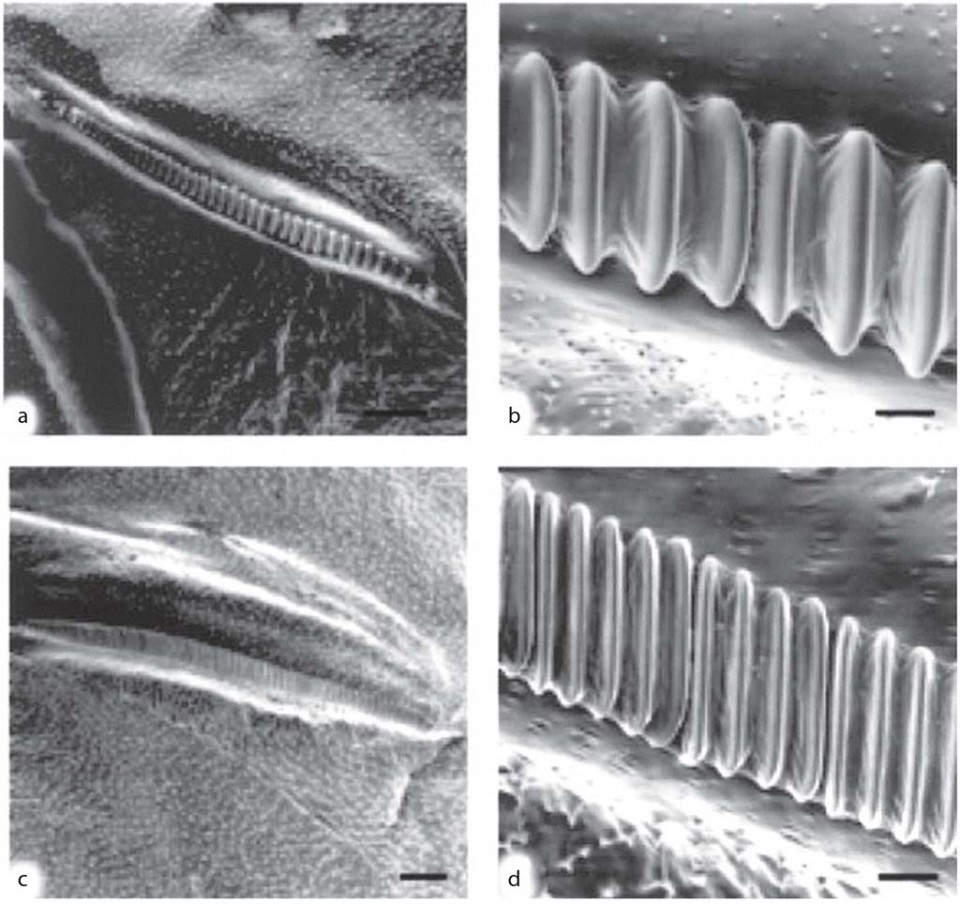
Figure 9 Interspecific differences in the stridulatory devices found in females of the burrower bugs Scaptocoris castanea and S. carvalhoi (Hemiptera: Cydnidae). Images a and b are S. castanea; images c and d are S. carvalhoi. Images a and c show the stridulitrum in the postcubital vein of the hind wings (scale bar = 100 μ); images b and d show the central section of the stridulitrum, showing details of the teeth (scale bar = 10 μ).
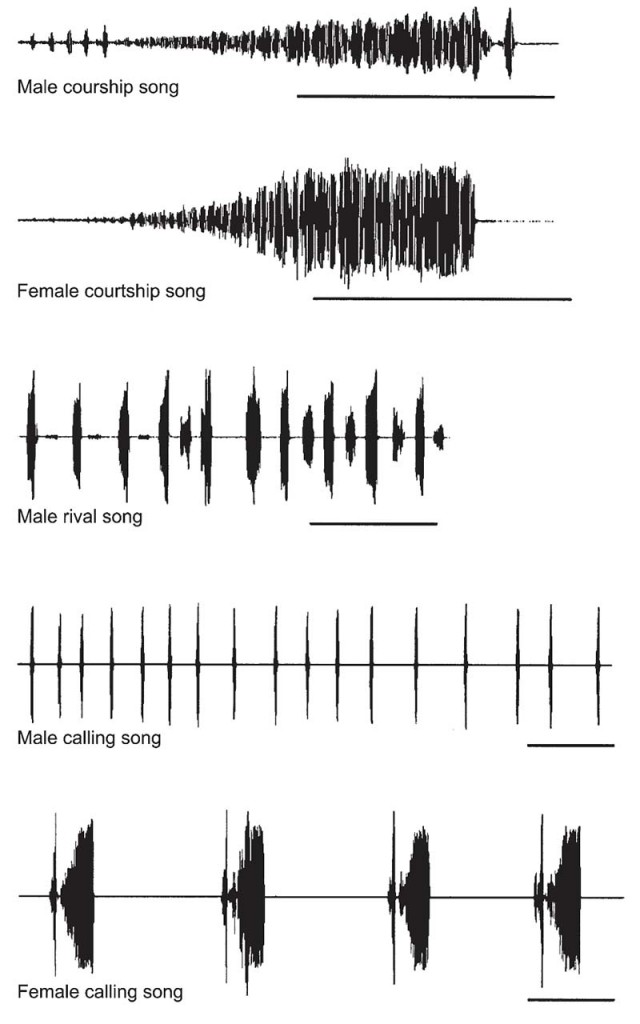
Figure 10 Oscillograms of songs emitted by males and females of the southern green stink bug, Nezara viridula (Pentatomidae).
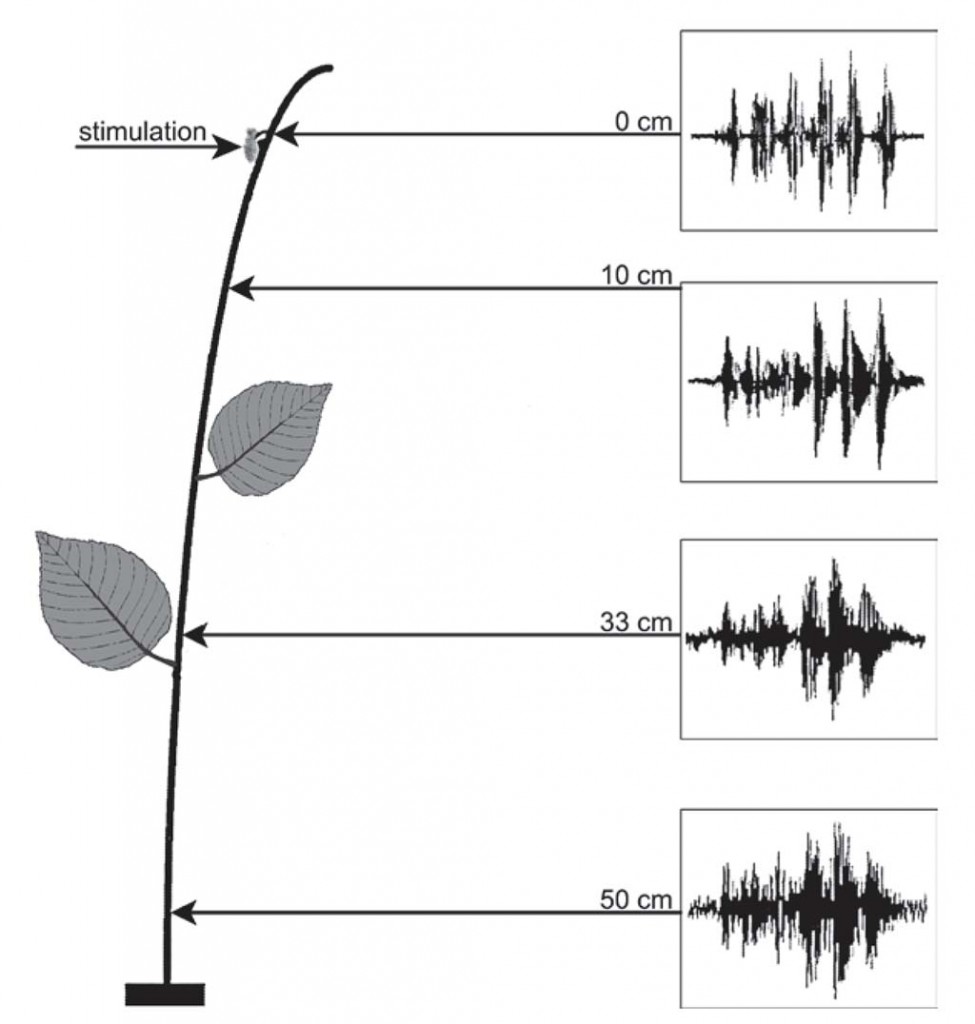
Figure 11 Laser vibrometer recordings taken from a plant fed upon by Nezara viridula showing the pattern of recordings at various distances (in cm) from the point of stimulation.
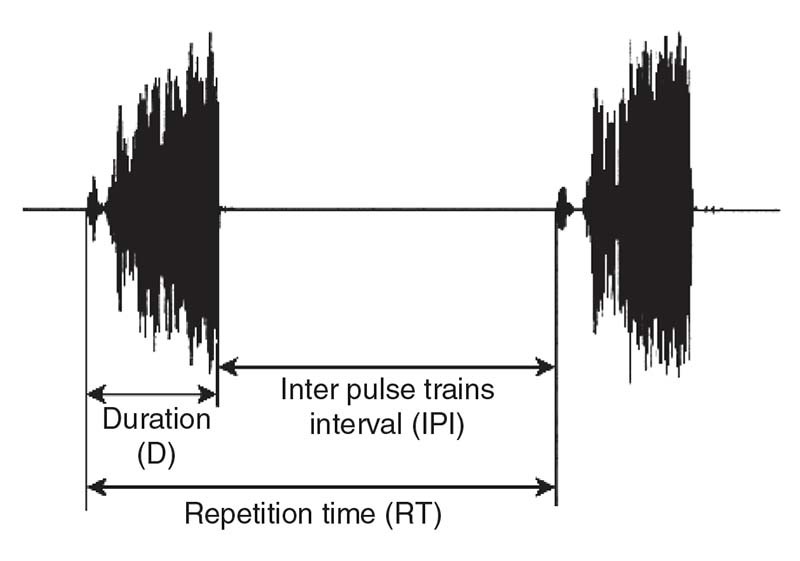
Figure 12 Temporal parameters of a vibratory signal
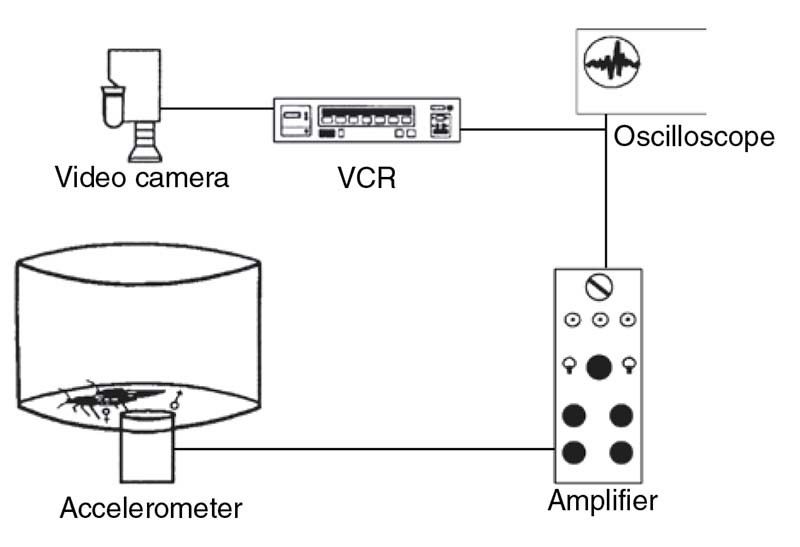
Figure 13 Experimental setupused to study substrate-borne signals produced by stridulation in Rhodnius prolixus (Hemiptera: Reduviidae). The accelerometer generated a signal with voltage proportional to the instantaneous acceleration of the moving object, electrical signals were amplified and monitored by an oscilloscope, then this information was stored in the sound track of a videotape. Also, the behavior of the bugs was simultaneously videotaped

Figure 14 Vibratory emissions of male and female burrower bugs Scaptocoris carvalhoi (above) and S. castanea (below) (Hemiptera: Cydnidae). A and B designate the two types of female song found in S. carvalhoi. Time scales are marked below oscillograms
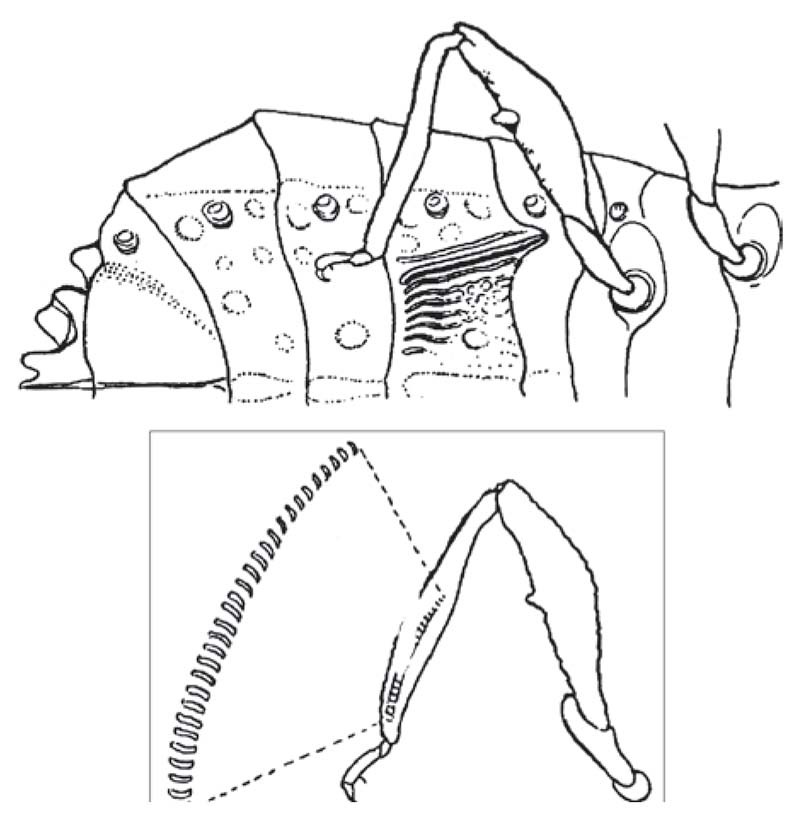
Figure 15 Artabanus lativentris (Hemiptera: Aradidae): (above) ventral view of abdomen, with file (stridulitrum), (below) hind leg with detail of scraper (plectrum) in the interior surface of the hind tibia
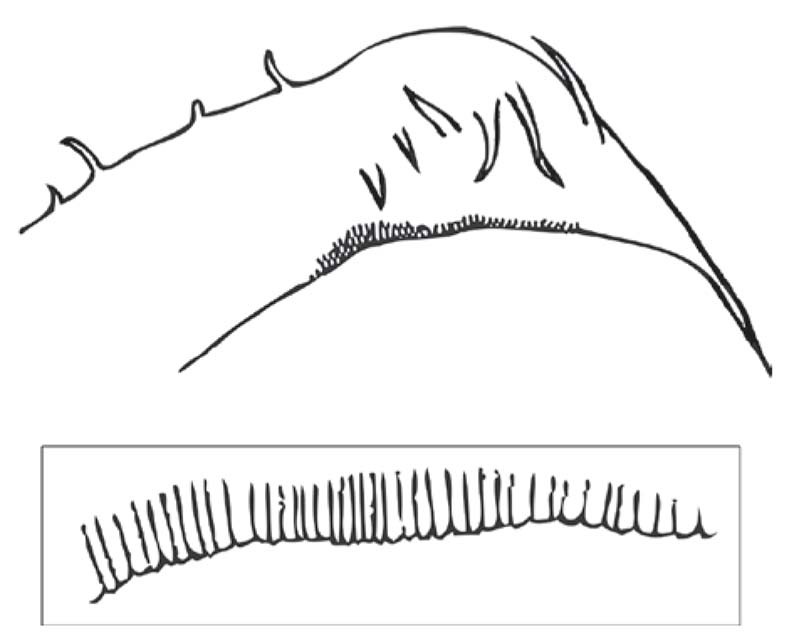
Figure 16 Phyllomorpha laciniata (Hemiptera: Coreidae): (above) dorsal view of pronotum with scraper (plectrum) at its margin, (below) detail of spines of scraper
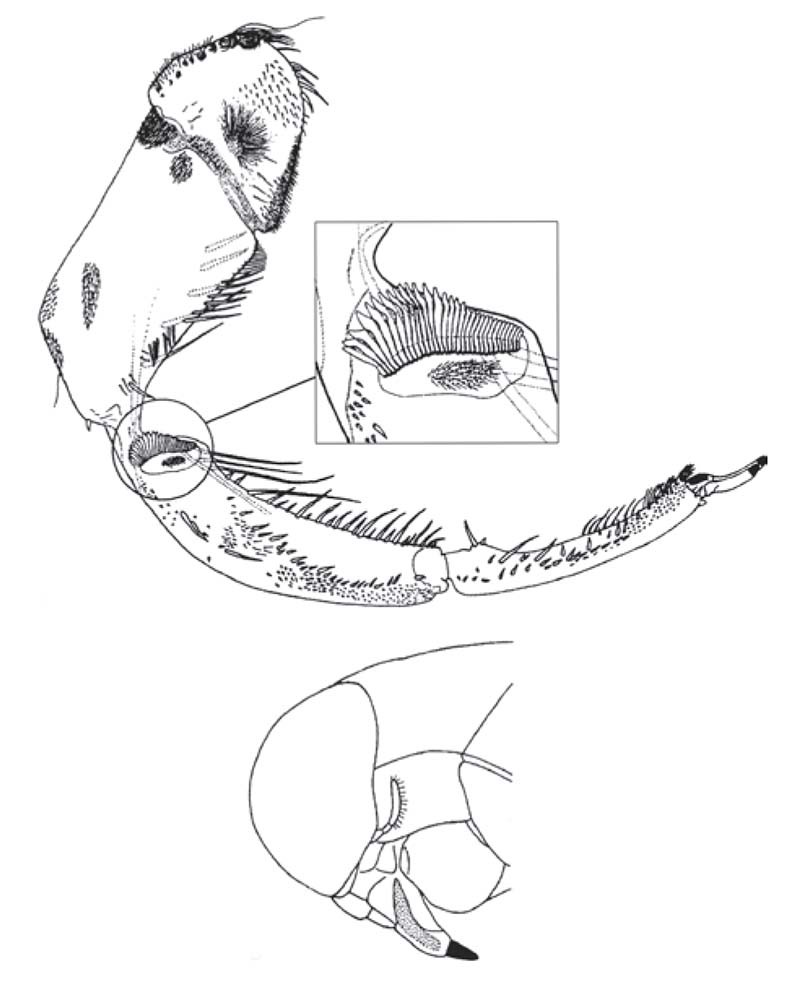
Figure 17 Male Anisops megalops (Hemiptera: Notonectidae): (above) foreleg with detail of scraper (plectrum) in the interior surface of the fore-tibia, (below) lateral view of the head with file (stridulitrum) on the labium

Acetylcholine Esterase


